Chemistry:Lansoprazole
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /lænˈsoʊprəzoʊl/ lan-SOH-prə-zohl |
| Trade names | Prevacid, others |
| Other names | AG 1749 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695020 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| Drug class | Proton pump inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80% or more |
| Protein binding | 97% |
| Metabolism | Liver (CYP3A4- and CYP2C19-mediated) |
| Elimination half-life | 1.0–1.5 hours |
| Excretion | Kidney and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
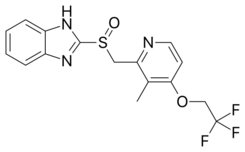
| Formula | C16H14F3N3O2S |
| Molar mass | 369.36 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| Melting point | 178 °C (352 °F) (decomposes) |
| |
| |
| (verify) | |
Lansoprazole, sold under the brand name Prevacid among others, is a medication which reduces stomach acid.[3] It is a proton pump inhibitor (PPI), used to treat peptic ulcer disease, gastroesophageal reflux disease, and Zollinger–Ellison syndrome.[4] Its effectiveness is similar to that of other PPIs.[5] It is taken by mouth.[3] Onset is over a few hours and effects last up to a couple of days.[3]
Common side effects include constipation, abdominal pain, and nausea.[3][2] Serious side effects may include osteoporosis, low blood magnesium, Clostridium difficile infection, and pneumonia.[3][2] Use in pregnancy and breastfeeding is of unclear safety.[1] It works by blocking H+/K+-ATPase in the parietal cells of the stomach.[3]
Lansoprazole was patented in 1984 and came into medical use in 1992.[6] It is available as a generic medication.[4] In 2020, it was the 191st most commonly prescribed medication in the United States, with more than 2 million prescriptions.[7][8]
Medical uses
Lansoprazole is used for treatment of:[2]
- Ulcers of the stomach and duodenum, and NSAID-induced ulcers
- Helicobacter pylori infection, alongside antibiotics (adjunctive treatment), treatment to kill H. pylori causing ulcers or other problems involves using two other drugs besides lansoprazole known as "triple therapy", and involves taking twice daily for 10 or 14 days lansoprazole, amoxicillin, and clarithromycin
- Gastroesophageal reflux disease
- Zollinger–Ellison syndrome[9]
There is no good evidence that it works better than other PPIs.[5]
Side effects
Side effects of PPIs in general[10] and lansoprazole in particular[11] may include:[2]
- Common: diarrhea, abdominal pain[12]
- Infrequent: dry mouth, insomnia, drowsiness, blurred vision, rash, pruritus
- Rarely and very rarely: taste disturbance, liver dysfunction, peripheral oedema, hypersensitivity reactions (including bronchospasm, urinary, angioedema, anaphylaxis), photosensitivity, fever, sweating, depression, interstitial nephritis, blood disorders (including leukopenia, leukocytosis, pancytopenia, thrombocytopenia), arthralgia, myalgia, skin reactions[13] including (erythroderma,[14] Stevens–Johnson syndrome, toxic epidermal necrolysis, bullous eruption)
PPIs may be associated with a greater risk of hip fractures and Clostridium difficile-associated diarrhea.[2]: 22
Interactions
Lansoprazole interacts with several other drugs, either due to its own nature or as a PPI.[15]
- PPIs reduce absorption of antifungals (itraconazole and ketoconazole) [16] and possibly increase digoxin in plasma
- Increases plasma concentrations of cilostazol (risk of toxicity)
Lansoprazole possibly interacts with, among other drugs:
- sucralfate
- ampicillin
- bisacodyl
- clopidogrel
- delavirdine
- fluvoxamine
- iron salts
- voriconazole
- aminophylline and theophylline
- astemizole
Chemistry
It is a racemic 1:1 mixture of the enantiomers dexlansoprazole and levolansoprazole.[17] Dexlansoprazole is an enantiomerically pure active ingredient of a commercial drug as a result of the enantiomeric shift. Lansoprazole's plasma elimination half-life (1.5 h) is not proportional to the duration of the drug's effects to the person (i.e. gastric acid suppression).[18]
History
Lansoprazole was originally synthesized at Takeda and was given the development name AG 1749.[19] Takeda patented it in 1984 and the drug launched in 1991.[20] In the United States, it was approved for medical use in 1995.[21]
Society and culture

Patents
Patent protection of the lansoprazole molecule expired on 10 November 2009,[22][23] and generic formulations became available under many brand names in many countries.[24] Some formulations may not be available in generic form.[25]
Availability
Since 2009, lansoprazole has been available over the counter (OTC) in the U.S. as Prevacid 24HR[26][27] and as Lansoprazole 24HR.[28] In Australia, it is marketed by Pfizer as Zoton.[29]
Research
In vitro experiments have shown that lansoprazole binds to the pathogenic form of tau protein.[30] As of 2015[update] laboratory studies were underway on analogs of lansoprazole to explore their use as potential PET imaging agents for diagnosing tauopathies including Alzheimer's disease.[30]
References
- ↑ 1.0 1.1 "Lansoprazole Use During Pregnancy". https://www.drugs.com/pregnancy/lansoprazole.html.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 "Lansoprazole capsule, delayed release pellets". DailyMed. U.S. National Library of Medicine. 11 October 2016. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c8fa7401-907f-41b6-a2e1-f4bc214f2cdf.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 "Lansoprazole Monograph for Professionals". American Society of Health-System Pharmacists. https://www.drugs.com/monograph/lansoprazole.html.
- ↑ 4.0 4.1 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 79–80. ISBN 9780857113382.
- ↑ 5.0 5.1 "Comparative effectiveness of proton pump inhibitors". Therapeutics Initiative. Dept of Anesthesiology, Pharmacology & Therapeutics, Faculty of Medicine, University of British Columbia. 28 June 2016. http://www.ti.ubc.ca/2016/06/28/99-comparative-effectiveness-proton-pump-inhibitors/.
- ↑ Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 445. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA445.
- ↑ "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Lansoprazole - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Lansoprazole.
- ↑ "Long-term treatment with lansoprazole for patients with Zollinger-Ellison syndrome". Alimentary Pharmacology & Therapeutics 10 (4): 507–522. August 1996. doi:10.1046/j.1365-2036.1996.10152000.x. PMID 8853754.
- ↑ "1.3.5 Proton pump inhibitors". British National Formulary. http://bnf.org/bnf/bnf/current/2137.htm#_200909.
- ↑ "Lansoprazole". British National Formulary. http://bnf.org/bnf/bnf/current/129429.htm#_129429.
- ↑ "Prevacid (Lansoprazole) Drug Information: Side Effects and Drug Interactions - Prescribing Information". http://www.rxlist.com/prevacid-drug/side-effects-interactions.htm.
- ↑ "Dermatologic adverse reactions to proton-pump inhibitors: A synthetized review". Journal of Cosmetic Dermatology 20 (4): 1073–1079. April 2021. doi:10.1111/jocd.13763. PMID 33031621.
- ↑ "Erythroderma". Dermatology. St. Louis: Mosby. 2007. p. 154. ISBN 978-1-4160-2999-1..
- ↑ "Lansoprazole interactions". British National Formulary. http://bnf.org/bnf/bnf/current/41001i753.htm.
- ↑ "Effects of ranitidine and sucralfate on ketoconazole bioavailability". Antimicrobial Agents and Chemotherapy 35 (9): 1765–1771. September 1991. doi:10.1128/aac.35.9.1765. PMID 1952845.
- ↑ "Pharmacy Benefit Update". http://epharmacytechnicanschools.com/pharmacy-benefit-update/.
- ↑ "Prevacid Pharmacology, Pharmacokinetics, Studies, Metabolism". RxList.com. 2007. http://www.rxlist.com/cgi/generic/lansop_cp.htm.
- ↑ Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 102. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA102.
- ↑ Drug Discovery and Development, Volume 1: Drug Discovery. John Wiley & Sons. 2006. p. 201. ISBN 9780471780090. https://books.google.com/books?id=Bu5IHnBxjxwC&pg=PA201.
- ↑ "Lansoprazole". Mosby's Drug Consult. http://www.mosbysdrugconsult.com/DrugConsult/Top_200/Drugs/e3230.html.
- ↑ "Prevacid Drug Profile". Drugpatentwatch.com. https://www.drugpatentwatch.com/p/tradename/index.php?query=PREVACID&archive=2008.
- ↑ "Teva to release Prevacid version when patent expires". Market Watch. Dow Jones. 7 November 2008. http://www.marketwatch.com/story/teva-to-release-prevacid-version-when-patent-expires.
- ↑ "International availability of lansoprazole". Drugs.com. https://www.drugs.com/international/lansoprazole.html.
- ↑ "Generic lansoprazole". Drugs.com. https://www.drugs.com/availability/generic-prevacid.html.
- ↑ "Prevacid 24 HR- lansoprazole capsule, delayed release". U.S. National Library of Medicine. 7 August 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=fd4629d5-b876-4ae9-bb32-c3560ad416a9.
- ↑ "Prevacid 24 HR- lansoprazole capsule, delayed release". DailyMed. U.S. National Library of Medicine. 11 December 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=99a97004-e247-4f4d-a38c-f28f521de1c4.
- ↑ "Lansoprazole 24 HR- lansoprazole capsule, delayed release". DailyMed. U.S. National Library of Medicine. 21 December 2017. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=b5b4e61e-91c5-41bd-a16d-e25d9be2c87e.
- ↑ "Zoton FasTabs" (in en). 2 September 2019. https://www.nps.org.au/medicine-finder/zoton-fas-tabs-orally-disintegrating-tablets.
- ↑ 30.0 30.1 "Tau imaging: early progress and future directions". The Lancet. Neurology 14 (1): 114–124. January 2015. doi:10.1016/s1474-4422(14)70252-2. PMID 25496902.
External links
- "Lansoprazole". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/lansoprazole.
 |
