Chemistry:Triethylgallium
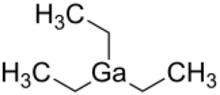
| |
| Names | |
|---|---|
| IUPAC name
triethylgallane
| |
| Systematic IUPAC name
triethylgallium | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| Properties | |
| C6H15Ga | |
| Molar mass | 156.9 g/mol |
| Appearance | colourless liquid |
| Melting point | −82.3 °C (−116.1 °F; 190.8 K) |
| Boiling point | 143 °C (289 °F; 416 K) |
| Reacts[1] | |
| Hazards | |
| Main hazards | pyrophoric |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Triethylgallium is the organogallium compound with the formul Ga(C2H5)3. Also called TEGa, it is a metalorganic source of gallium for metalorganic vapour phase epitaxy (MOVPE) of compound semiconductors. It is a colorless pyrophoric liquid,[2] typically handled with air-free techniques.
Preparation and reactions
The main routes involve alkylation of gallium trichloride. When this alkylation is effected with ethyl Grignard reagent in ether, the product is the diethyl ether adduct of triethylgallium. The ether is not easily removed. Thus an alternative route involves transmetalation with triethylaluminium according to this simplified equation:[3]
- GaCl
3 + 3 AlEt
3 → GaEt
3 + 3 AlClEt
2
Triethylgallium readily converts to the air-stable, colorless alkoxide by two routes, oxidation and alcoholysis:[3]
- GaEt
3 + 0.5 O
2 → GaEt
2(OEt) - GaEt
3 + EtOH → GaEt
2(OEt) + EtH
The sweet odor associated with triethylgallium is due to the alkoxide.
Redistribution reactions occur with gallium trichloride:[3]
- 2GaEt
3 + GaCl
3 → 3 GaEt
2Cl
Applications
TEGa can be a useful alternative to trimethylgallium in the metalorganic vapour phase epitaxy of compound semiconductors because films grown using TEGa have been shown to have a lower carbon impurity concentration.[4]
Related compounds
- Trimethylgallium, with similar properties.
References
- ↑ amdg.ece.gatech.edu/msds/mo/teg_epichem.pdf
- ↑ Shenaikhatkhate, D; Goyette, R; Dicarlojr, R; Dripps, G (2004). "Environment, health and safety issues for sources used in MOVPE growth of compound semiconductors". Journal of Crystal Growth 272: 816. doi:10.1016/j.jcrysgro.2004.09.007. Bibcode: 2004JCrGr.272..816S.
- ↑ 3.0 3.1 3.2 J.J.Eisch, R. B. King, ed (1981). Organometallic Syntheses Volume 2. Nontransition Metal Compounds. NY, NY: Academic Press.
- ↑ Saxler, A; Walker, D; Kung, P; Zhang, X; Razeghi, M; Solomon, J; Mitchel, W; Vydyanath, H (1997). "Comparison of trimethylgallium and triethylgallium for the growth of GaN". Applied Physics Letters 71: 3272. doi:10.1063/1.120310. Bibcode: 1997ApPhL..71.3272S. https://zenodo.org/record/1231844/files/article.pdf.
 |
