Chemistry:Xenic acid
From HandWiki
|
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
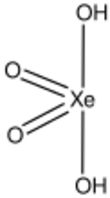
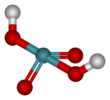
| H2XeO4 | |||
| Molar mass | 197.31 g/mol | ||
| Related compounds | |||
Related compounds
|
Perxenic acid Xenon trioxide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Xenic acid is a proposed noble gas compound with the chemical formula H2XeO4 or XeO2(OH)2. It has not been isolated, and the published characterization data are ambiguous.[1]
Salts of xenic acid are called xenates, containing the HXeO−4 anion, such as monosodium xenate. They tend to disproportionate into xenon gas and perxenates:[2]
- 2 HXeO−4 + 2 OH− → XeO4−6 + Xe + O2 + 2 H2O
The energy given off is sufficient to form ozone from diatomic oxygen:
- 3 O2 (g) → 2 O3 (g)
Salts containing the deprotonated anion XeO2−4 are presently unknown.[2]
References
- ↑ Claassen, Howard H.; Knapp, Geraldine. (1964). "Raman Spectrum of Xenic Acid". Journal of the American Chemical Society 86 (12): 2341–2342. doi:10.1021/ja01066a008.
- ↑ 2.0 2.1 Egon Wiberg; Nils Wiberg; Arnold Frederick Holleman (2001). Inorganic chemistry. Academic Press. p. 399. ISBN 0-12-352651-5.
Further reading
- Bruno Jaselskis, Stanislaus Vas (May 1964). "Xenic Acid Reactions with vic-Diols". J. Am. Chem. Soc. 86 (10): 2078–2079. doi:10.1021/ja01064a041.
 |