Chemistry:Xenon tetrachloride
From HandWiki
Short description: Chemical compound
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
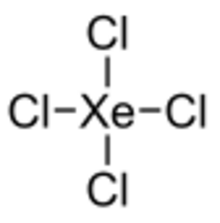
| Cl4Xe | |
| Molar mass | 273.09 g·mol−1 |
| Related compounds | |
Related compounds
|
XeF4, XeCl2, XeCl |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Xenon tetrachloride is an unstable[1] inorganic compound with the chemical formula XeCl4. Unlike other noble gas/halide compounds, it cannot be synthesized by simply combining the elements, by using a more-active halogenating agent, or by substitution of other halides on tetrahaloxenon compounds. Instead, a decay technique can be used, starting with K129ICl4. The iodine-129 atom of the 129ICl–4 covalent cluster is radioactive and undergoes beta decay to become xenon-129.[2][3] The resulting XeCl4 molecule has a square planar molecular geometry analogous to xenon tetrafluoride.[4]
Alternately, the product can be obtained by subjecting the elements to an electric discharge.[1]
References
- ↑ 1.0 1.1 Holleman, A.F.; Wiberg, E.; Wiberg, N.; Eagleson, M.; Brewer, W. (2001). Inorganic Chemistry. Academic Press. p. 394. ISBN 9780123526519.
- ↑ Bell, C.F. (2013). Syntheses and Physical Studies of Inorganic Compounds. Elsevier Science. p. 143. ISBN 9781483280608.
- ↑ Cockett, A.H.; Smith, K.C.; Bartlett, N. (2013). The Chemistry of the Monatomic Gases: Pergamon Texts in Inorganic Chemistry. Elsevier Science. p. 292. ISBN 9781483157368.
- ↑ Perlow, G. J.; Perlow, M. R. (15 August 1964). "Mössbauer Effect Evidence for the Existence and Structure of XeCl4". The Journal of Chemical Physics 41 (4): 1157–1158. doi:10.1063/1.1726022. Bibcode: 1964JChPh..41.1157P.
 |

