Chemistry:Xenon dichloride
From HandWiki
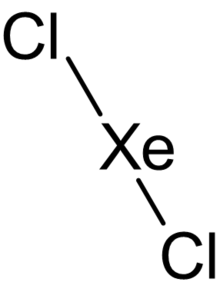
| |
| Names | |
|---|---|
| IUPAC name
Dichloroxenon
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| XeCl2 | |
| Molar mass | 202.199 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Xenon dichloride (XeCl2) is a xenon compound and the only known stable chloride of xenon. The compound can be prepared by using microwave discharges towards the mixture of xenon and chlorine, and it can be isolated from a condensate trap. One experiment[which?] tried to use xenon, chlorine and boron trichloride to produce XeCl2·BCl3, but only generated xenon dichloride.[1]
However, it is still doubtful whether xenon dichloride is a true compound or a Van der Waals molecule composed of a xenon atom and a chlorine molecule connected by a secondary bond.[2]
References
- ↑ 张青莲 [Zhang Qingliang] (November 1991) (in zh-hans). 无机化学丛书 [Inorganic Chemistry]. 1. Beijing: Science Press. pp. 72. ISBN 7-03-002238-6.
- ↑ Proserpio, Davide M.; Hoffmann, Roald; Janda, Kenneth C. (1991). "The xenon-chlorine conundrum: van der Waals complex or linear molecule?". Journal of the American Chemical Society 113 (19): 7184. doi:10.1021/ja00019a014.
 |

