Chemistry:Xenon dioxide
| |
| Names | |
|---|---|
| IUPAC name
xenon dioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| |
| |
| Properties | |
| XeO2 | |
| Molar mass | 163.29 g/mol |
| Appearance | yellow solid[1] |
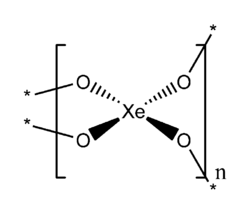
| Structure | |
| Bent | |
| Related compounds | |
Related compounds
|
Xenon trioxide Xenon tetroxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Xenon dioxide, or xenon(IV) oxide, is a compound of xenon and oxygen with formula XeO2 which was synthesized in 2011. It is synthesized at 0 °C by hydrolysis of xenon tetrafluoride in aqueous sulfuric acid:[2] [math]\ce{ XeF4 + 2H2O -> XeO2 + 4HF }[/math]
Structure
XeO2 has an extended (chain or network) structure in which xenon and oxygen have coordination numbers of four and two respectively. The geometry at xenon is square planar, consistent with VSEPR theory for four ligands and two lone pairs (or AX4E2 in the notation of VSEPR theory).
In addition, the existence of an XeO2 molecule was predicted by an ab initio quantum chemistry method several years earlier by Pyykkö and Tamm, but these authors did not consider an extended structure.[3]
Properties
XeO2 is a yellow-orange solid.[4] It is an unstable compound, with a half-life of about two minutes, disproportionating into XeO3 and xenon gas. Its structure and identity was confirmed by cooling it to −78 °C so that Raman spectroscopy could be performed before it decomposed.[1]
- 3 XeO2 → Xe + 2 XeO3
References
- ↑ 1.0 1.1 Tyler Irving (May 2011). "Xenon Dioxide May Solve One of Earth's Mysteries". L’Actualité chimique canadienne (Canadian Chemical News). http://www.accn.ca/index.php?ci_id=2583&la_id=1.
- ↑ Brock, David S.; Schrobilgen, Gary J. (2011). "Synthesis of the Missing Oxide of Xenon, XeO2, and Its Implications for Earth's Missing Xenon". Journal of the American Chemical Society 133 (16): 6265–6269. doi:10.1021/ja110618g. PMID 21341650.
- ↑ Pyykkö, Pekka; Tamm, Toomas (1 April 2000). "Calculations for XeOn(n = 2−4): Could the Xenon Dioxide Molecule Exist?". The Journal of Physical Chemistry A 104 (16): 3826–3828. doi:10.1021/jp994038d.
- ↑ Cotton, Simon (1 May 2011). "Xenon dioxide". Education in Chemistry (Royal Society of Chemistry) 48 (3): 69. https://eic.rsc.org/soundbite/xenon-dioxide/2021269.article. Retrieved 2012-05-18.
 |

