Chemistry:Nitrosonium octafluoroxenate(VI)
From HandWiki
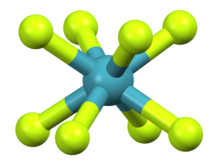 The square antiprismatic [XeF8]2− anion in (NO)2XeF8[1]
| |||
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Nitrosonium octafluoroxenate(VI)
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| |||
| |||
| Properties | |||
| (NO)2XeF8 | |||
| Density | 3.354 g/cm3 | ||
| Reacts | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Nitrosonium octafluoroxenate(VI) is a chemical compound of xenon with nitrogen, oxygen, and fluorine, having formula (NO)2XeF8. It is an ionic compound containing well-separated nitrosonium cations (NO+) and octafluoroxenate(VI) anions (XeF2−8). The molecular geometry of the octafluoroxenate(VI) ion is square antiprismatic, having Xe–F bond lengths of 1.971 Å, 1.946 Å, 1.958 Å, 2.052 Å, and 2.099 Å.[1]
It is synthesized by the reaction of xenon hexafluoride (XeF6) with nitrosyl fluoride (NOF):[2]
- XeF6 + 2 NOF → (NO)2XeF8
Other compounds containing the octafluoroxenate(VI) ion include its alkali metal salts, including Cs2XeF8 and Rb2XeF8, which are stable up to 400 °C.[3][4]
References
- ↑ 1.0 1.1 Peterson, W.; Holloway, H.; Coyle, A.; Williams, M. (Sep 1971). "Antiprismatic Coordination about Xenon: the Structure of Nitrosonium Octafluoroxenate(VI)". Science 173 (4003): 1238–1239. doi:10.1126/science.173.4003.1238. ISSN 0036-8075. PMID 17775218. Bibcode: 1971Sci...173.1238P.
- ↑ Cotton (2007). Advanced Inorganic Chemistry (6th ed.). Wiley-India. p. 588. ISBN 978-81-265-1338-3.
- ↑ Chandra, Sulekh (2004). Comprehensive Inorganic Chemistry. New Age International. p. 308. ISBN 81-224-1512-1.
- ↑ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
 |


