Medicine:Chronic cerebrospinal venous insufficiency controversy
| Chronic cerebrospinal venous insufficiency | |
|---|---|
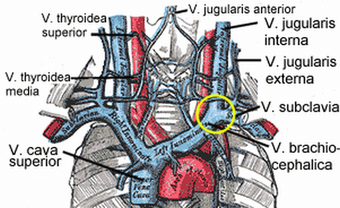 | |
| Veins of the neck. V.jugularis interna is proposed to be stenosed or have a malformed valve in CCSVI cases. |
Chronic cerebrospinal venous insufficiency (CCSVI or CCVI) is a term invented by Italian researcher Paolo Zamboni in 2008 to describe compromised flow of blood in the veins draining the central nervous system.[1][2] Zamboni hypothesized that it might play a role in the cause or development of multiple sclerosis (MS).[3][4] Zamboni also devised a surgical procedure which the media nicknamed a liberation procedure or liberation therapy, involving venoplasty or stenting of certain veins.[5] Zamboni's ideas about CCSVI are very controversial, with significantly more detractors than supporters, and any treatments based on his ideas are considered experimental.[6][7]
There is no scientific evidence that CCSVI is related to MS, and there is no good evidence that the surgery helps MS patients. Zamboni's first published research was neither blinded nor did it have a comparison group.[5] Zamboni also did not disclose his financial ties to Esaote, the manufacturer of the ultrasound specifically used in CCSVI diagnosis.[8] The "liberation procedure" has been criticized for possibly resulting in serious complications and deaths, while its purported benefits have not been proven.[5][7] In 2012, the United States Food and Drug Administration states that it is not clear if CCSVI exists as a clinical entity and that these treatments may cause more harm.[9] In 2017 they emphasized that this use of balloon angioplasty is not an approved use.[10] In a 2017 study Zamboni et al. stated "Venous PTA cannot be recommended for patients with relapsing-remitting multiple sclerosis."[11] In 2018 a study in Neurology concluded "Our data do not support the continued use of venoplasty of extracranial jugular and/or azygous venous narrowing to improve patient-reported outcomes, chronic MS symptoms, or the disease course of MS."[12]
Research on CCSVI was fast-tracked, but researchers have been unable to find a connection between CCSVI and MS.[13] This has raised serious objections to the hypothesis of CCSVI originating multiple sclerosis.[14] Additional research investigating the CCSVI hypothesis is underway.[15] A 2013 study found that CCSVI is equally rare in people with and without MS, while narrowing of the cervical veins is equally common.[16][17]
Hypothesis
Proposed consequences of CCSVI syndrome include intracranial hypoxia, delayed perfusion, reduced drainage of catabolites, increased transpulmonary pressure,[18] and iron deposits around the cerebral veins.[19][20] Multiple sclerosis has been proposed as a possible outcome of CCSVI.[citation needed]
Pathophysiology
Zamboni and colleagues claimed that in MS patients diagnosed with CCSVI, the azygos and IJV veins are stenotic (abnormally narrowed) in around 90% of cases. Zamboni theorized that malformed blood vessels cause increased deposition of iron in the brain, which in turn triggers autoimmunity and degeneration of the nerve's myelin sheath.[19][21] While the initial article on CCSVI claimed that abnormal venous function parameters were not seen in healthy people, others have noted that this is not the case.[21] In the report by Zamboni none of the healthy participants met criteria for a diagnosis of CCSVI while all patients did.[1][21] Such outstanding results have raised suspicions of a possible spectrum bias, which originates on a diagnostic test not being used under clinically significant conditions.[21]
Further studies of the relationship between CCSVI and MS have had variable results,[13] with many failing to reproduce the association between MS and CCSVI.[22][23][24] Moreover, the greatest predictor of positive results is researchers' involvement in the administration of the "liberation procedure".[22][24] This effect goes to the extent that, when only fully independent studies are considered, no association at all is found.[24] The poor reproducibility across studies and diagnostic modalities has led some authors to conclude that CCVSI might be nothing more than a clinically irrelevant sonographic construct.[22]
Already by 2010, there were "a growing number of papers that raise serious questions about its (CCSVI) validity",[14] although evidence had been "both for and against the controversial hypothesis".[25] It was agreed that it was urgent to perform appropriate epidemiological studies to define the possible relationship between CCSVI and MS, although existing data did not support CCSVI as the cause of MS.[13]
Venous malformations
Most of the venous problems in MS patients have been reported to be truncular venous malformations, including azygous stenosis, defective jugular valves and jugular vein aneurysms. Problems with the innominate vein and superior vena cava have also been reported to contribute to CCSVI.[26] A vascular component in MS had been cited previously.[27][28]
Several characteristics of venous diseases make it difficult to include MS in this group.[14] In its current form, CCSVI cannot explain some of the epidemiological findings in MS. These include risk factors such as Epstein-Barr infection, parental ancestry, date of birth and geographic location.[14][29] MS is also more common in women, while venous diseases are more common in men. Venous pathology is commonly associated with hypertension, infarcts, edema and transient ischemia, and occurs more often with age, however these conditions are hardly ever seen in MS and the disease seldom appears after age 50. Finally, an organ-specific immune response is not seen in any other kind of venous disease.[14]
Iron deposits
Iron deposition as a cause of MS received support when a relationship between venous pressure and iron depositions in MS patients was found in a neuroimaging study, and criticism as other researchers found normal ferritin levels in the cerebrospinal fluid of MS patients.[13][30] Additionally iron deposition occurs in different neurological diseases such as Alzheimer's disease or Parkinson's disease that are not associated with CCSVI.[1][21] Evidence linking CCSVI and iron deposition is lacking, and dysregulation of iron metabolism in MS is more complex than simply iron accumulation in the brain tissue.[31]
Genetics
A small genetic study looked at fifteen MS patients who also had CCSVI. It found 234 specific copy number variations in the human leukocyte antigen focus. Of these, GRB2, HSPA1L and HSPA1A were found to be specifically connected to both MS and angiogenesis, TAF11 was connected to both MS and artery passage, and HLA-DQA2 was suggestive of having an implication for angiogenesis as it interacts with CD4.[32] A study in 268 MS patients and 155 controls reported more a frequency of CCSVI in the MS group that was more than twice as high as in the controls group and was also higher in the progressive MS group than in the non-progressive MS group. This study found no relationship between CCSVI and HLA DRB1*1501, a genetic variation that has been consistently linked to MS.[33]
Diagnosis
CCSVI was first described using specialized extracranial and transcranial doppler sonography.[1][21] Five ultrasound criteria of venous drainage have been proposed to be characteristic of the syndrome, although two are considered sufficient for diagnosis of CCSVI:[1][21][34]
- reflux in the internal jugular and vertebral veins,
- reflux in the deep cerebral veins,
- high-resolution B-mode ultrasound evidence of stenosis of the internal jugular vein,
- absence of flow in the internal jugular or vertebral veins on Doppler ultrasound, and
- reverted postural control of the main cerebral venous outflow pathways.
It is still not clear whether magnetic resonance venography, venous angiography, or Doppler sonography should be considered the gold standard for the diagnosis of CCSVI.[13] Use of magnetic resonance venography for the diagnosis of CCSVI in MS patients has been proposed by some to have limited value, and should be used only in combination with other techniques.[35] Others have stated that magnetic resonance venography is a valid measure which has advantages over Doppler including the fact that results are more operator-independent.[36]
Diagnostic criteria have been criticized. Both the number of criteria and the need of being positive for two of them as enough for diagnosis are arbitrary ideas.[36] Moreover, experienced groups in the use of ultrasound have not been able to show intracranial or extracranial reflux in MS patients or even healthy controls whereas the criterion of absence of flow and the criterion regarding stenosis are considered not valid since they are related to normal physiological processes and not pathology.[36] These problems in the criteria have led some researchers to consider the criteria inadequate and more generally the concept of CCSVI flawed.[36]
Treatment
All proposed treatments are experimental
Treatment based on the idea of CCSVI is considered experimental.[6]
Further trials are required to determine if the benefits, if any, of the procedure outweigh its risks.[21] Most experts, and medical and patients organizations, including the National Multiple Sclerosis Society of the USA or the Cardiovascular and Interventional Radiological Society of Europe (CIRSE), recommend not using the proposed treatment outside clinical trials until its effectiveness is confirmed by controlled studies.[3][5][7][21][37][38] Moreover, the CIRSE has stated that treatment research should begin by a small, placebo-controlled, prospective randomised trial which should be monitored by an independent organization.[38] An exception has been the Society of Interventional Radiology in the US and Canada, which considered research on the effectiveness of CCSVI intervention to be inconclusive as of 2010.[39] In March 2013 a press release indicated that the first prospective, placebo-controlled study of balloon angioplasty for MS had not shown any benefit of the therapy. The study, a phase II clinical trial designed to evaluate safety and efficacy of endovascular treatment, enrolled initially 10 patients that received the treatment and 20 more afterwards that were either allocated to receive angioplasty or a placebo intervention.[40]
Kuwait became the first country in the world where treatment of CCSVI, as of 2010, was explicitly allowed by the medical authorities and paid by the state health system.[41] As of 2010, the procedure was performed privately in 40 countries,[42] and, despite existing recommendations, as of 2013 it is believed that over 30,000 patients have undergone the procedure.[40]
Procedures
Balloon angioplasty and stenting have been proposed as treatment options for CCSVI in MS. The proposed treatment has been termed "liberation therapy" though the name has been criticized for suggesting unrealistic results.[14]
Balloon angioplasty in a preliminary, uncontrolled, unblinded study by Zamboni improved symptoms in MS in a minority of treated people.[43] Although the procedure pushes the vein open temporarily, the effect does not persist,[21] supporters advise against using stents.[44]
Venous percutaneous transluminal angioplasty (PTA) has proven to be safe but due to its ineffectiveness is not recommended.[15]
Adverse effects
While the procedure has been reported to be generally safe for MS patients,[13][40][45] severe complications related to the angioplasty and stenting that have been reported include intracranial hemorrhage, stent migration into a renal vein, thrombosis and nerve compression syndrome of cranial nerves XI and XII.[13][14][36] One death case appeared in the scientific literature, while 3 other deaths have been related to CCSVI treatment in the media.[36] Some United States hospitals have banned the surgical procedure outside clinical trials due to safety concerns until more evidence to support its use is available.[7][46]
In May 2012 the U.S. Food and Drug Administration issued a safety communication on CCSVI, stating that MS patients undergoing angioplasty and/or stenting to treat CCSVI risk serious injuries or death. Furthermore, it also noted that the benefits of these experimental procedures have not been proven and that studies exploring a link between MS and CCSVI are inconclusive.[9]
History
Venous pathology has been associated with MS for more than a century. Pathologist Georg Eduard Rindfleisch noted in 1863 that the inflammation-associated lesions were distributed around veins.[47] Later, in 1935, Tracy Putnam was able to produce similar lesions in dogs blocking their veins[48]
The term "chronic cerebrospinal venous insufficiency" was coined in 2008 by Paolo Zamboni, who described it in patients with multiple sclerosis. According to Zamboni, CCSVI had a high sensitivity and specificity differentiating healthy individuals from those with multiple sclerosis.[1][21] Zamboni's results were criticized because his study was not blinded and his results needed to be verified by further studies.[1][21] Zamboni had become interested in CCSVI in 1999 when his wife was diagnosed with MS.[49]
Society and culture
Conflict of interest
Paolo Zamboni has patents related to the highly sensitive ultrasound diagnostic systems manufactured by Esaote which, he proposes, should be used to diagnose CCSVI.[8] Moreover, Zamboni's research center has also received support in the form of equipment and technical assistance from this manufacturer.[8] These are potential conflicts of interest that he has never disclosed when publishing scientific articles, which would be against ethical practices of some countries such as the United States.[8]
Media
CCSVI has received a great deal of attention in all media, the scientific literature and on the Internet.[14] Moreover, the CCSVI case has been considered a good example of how new communication technologies and social media are modifying the traditional relationship between science, politics, medicine, and the general public.[49] In this sense they have played a key role in effectively promoting the theory.[49]
Media coverage has been perceived by some as "hype", with exaggerated claims that have led to excessive expectations.[5][50] This has been partially attributed to some of the investigators of the theory.[5] Mainstream media initial approximations to Zamboni's theory were enthusiastic and emphasized Zamboni's effort to find a cure for his wife, along with the improvement of some patients after its alleged treatment.[49] Initial difficulty reproducing results connecting MS and CCSVI, combined with reports of secondary effects of the surgical procedure, led to a more cautious discourse proposing that more investigation in the relationship between CCSVI and MS was needed.[49] The first fatality related to CCSVI treatment was a pivotal development in this trend towards a less optimistic media view of the theory.[49]
The Internet has been extensively used by patient groups to obtain and disseminate information on CCSVI. People with MS often read extensively about the CCSVI theory and its development on Internet sites,[51] and a search for "liberation procedure" in Google as of August 2010 yielded more than 2.5 million hits.[14] The Internet has also been used to advertise places where stenting for CCSVI is performed,[14] and to more generally disseminate all the information on CCSVI.[49] Social media have served patient groups in their attempt to pressure official bodies to make decisions favoring funding of clinical trials, or the public coverage of stenting and venoplasty as treatments of MS.[49] Likewise, social media have been accused of creating a division between CCSVI supporters and those who say it does not work.[49][50] Indeed, they have been repeatedly used by advocates of the CCSVI theory to attack those who were more critical or cautious, most commonly with accusations of being tainted due to commercial relationships with pharmaceutical companies.[49]
Many patients who have had the surgical procedure report their improvements on social media websites such as structured patient databases and YouTube.[36][51] Such stories are only anecdotal evidence of efficacy, and do not constitute a scientific proof of the efficacy of the treatment since, for example, those who have had a positive result are more prone to post their cases than those who had little or no improvement,[51] and the reported improvements in patients' condition can be attributed to the placebo effect.[50][52][53] Patients' reasons for not publishing negative results may include embarrassment about the money spent in the procedure without effect and the negative reaction they expect from other people with MS.[50] Caution has been recommended regarding patients' self-reports found on the web.[50][51][52]
Scientists and physicians transmitted their arguments regarding CCSVI inadequately, especially after the initial rising of the hypothesis. Their communication was characterized by an excessive hesitation and a lack of clear statements, as opposed to CCSVI proponents, who "won the communication battle, at least in the early rounds."[49] A positive effect of the important media coverage may be that it forces the world of medical research to be self-critical and give appropriate responses to the questions that globalization of the theory raises, especially among people with MS.[50]
Reception in Canada
While reasons are not completely clear, the CCSVI theory was received differently in Canada than other places. The public interest and number of media appearances were much greater than elsewhere, including Italy, and debate has been heated regarding funding.[25][49] As an example, by the end of 2009, a public petition to the country health authorities in support of the "liberation treatment" had received over 17,000 signatures.[49] The debate regarding funding in Canada has been considered to be specially informative as an example of extreme involvement of politics, due to public pressure, in decisions usually governed by scientific evidence.[49]
In 2009, the Multiple Sclerosis Society of Canada committed to funding research on the connection between CCSVI and MS,[54] although later in 2010 it came under criticism for opposing clinical trials of CCSVI therapy.[55] The MS Society of Canada in September 2010 reserved one million dollars toward CCSVI research "when a therapeutic trial is warranted and approved."[56]
At a political level there have been contradictory positions, with some provinces funding trials, others stating that since therapy is unproven they should wait,[57][58] and others urging for a pan-Canadian trial.[59] British Columbia, Alberta, and Newfoundland and Labrador funded observational studies in which patients who had already received the treatment were included. Over 2 million dollars were allocated to these studies.[49] The province of Saskatchewan was more aggressive and provided 2.2 million dollars for some of its residents to be included in a clinical trial.[49]
The Canadian Institutes of Health Research (CIHR), the federal agency responsible for funding health research, recommended in 2010 against funding a pan-Canadian trial of liberation therapy because there was a lack of evidence on the safety or efficacy of the procedure. It suggested a scientific expert working group made up of the principal investigators for the seven MS Society-sponsored studies.[49][60] The health minister accepted the CIHR recommendation and said that Canada was not going to fund clinical trials.[61] The expert panel was created by the end of 2010 together between the CIHR and the MS Society of Canada.[49] It has been proposed that the creation of this expert panel was partly directed to cope with the high levels of social pressure the CCSVI theory had raised and at the same time try to maintain a scientific perspective in the funding and investigation of CCSVI.[49] The main task of the panel was to monitor the results of the ongoing studies in the relationship between CCSVI and MS and recommend the funding of a clinical trial in case that there was evidence of a true relationship between the two.[49] In 2011, the Canadian federal government announced that they would fund clinical trials of the procedure to widen the veins since CIHR considered that evidence of venous abnormalities in MS was enough for small treatment trials.[49][62] It has been proposed that the recommendation to fund phase I and II trials instead of a big study was a compromise between the high levels of social and political pressure and the low level of evidence on the theory.[49]
Two qualitative studies have investigated the motives and experiences of Canadian patients traveling abroad to get the "liberation procedure".[63][64] One of the studies identified three factors contributing to patients going abroad seeking treatment: a loss of faith in the Canadian health system when it did not provide access to CCSVI treatment in Canada, hope in the new treatment as a solution for their worsening health, and trust in the MS community and the organizations, clinics and doctors facilitating or providing the desired operation.[63] Conversely, the other study concluded that sense of community and cooperation (from family, MS groups and the general population) was a key motivating factor.[64] Other motivating factors included media reports, perception of approval from their health providers, the apparent low risk of the operation, or accessibility of the hospital that offered the procedure directly or through a medical tourism company.[64] On the other hand, hesitating factors included the cost and effort required for the operation, the mistrust of foreign health systems, the underlying rationale for the operation, or advice against the procedure from trusted health providers.[64]
In 2013, a case-control study found evidence against the involvement of chronic cerebrospinal venous abnormalities in MS.[65] Later in 2013 a study found that vein narrowing appears to be present equally in those with and without MS on ultrasound and catheter venography.[66] The results of the study were described as a "death knell" for Zamboni's theory.[67] Another study released by the University of British Columbia in 2017 was described as a "definitive debunking" of liberation therapy.[68]
Organizations
Several national and international organizations have been created to further the research and dissemination of the CCSVI theory, such as the International Society for Neurovascular Disease and the National CCSVI Society of Canada.[69] They are working together with already existing organizations like the International Union of Phlebology (Union internationale de phlébologie-UIP- in French, its original working language)[70] of which Zamboni is a member.[71] The UIP for example proposed that developmental abnormalities were the primary cause of CCSVI.[72]
Research
There were further studies aimed at clarifying if there is a relationship between MS and CCSVI. In particular, the US and Canadian MS societies launched seven such studies.[14][73] Recent reviewers have shown "no significant difference in prevalence of CCSVI in people with MS compared to people without MS".[36] In 2014 imaging criteria for venous abnormalities were published to help with research on this topic.[74]
See also
- Chronic venous insufficiency
- Pathophysiology of multiple sclerosis
- Vascular myelopathy
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis". Journal of Neurology, Neurosurgery, and Psychiatry 80 (4): 392–9. April 2009. doi:10.1136/jnnp.2008.157164. PMID 19060024.
- ↑ "Internal jugular vein morphology and hemodynamics in patients with multiple sclerosis". International Angiology 29 (2): 115–20. April 2010. PMID 20351667.
- ↑ 3.0 3.1 "Chronic cerebrospinal venous insufficiency and multiple sclerosis". Annals of Neurology 67 (3): 286–90. March 2010. doi:10.1002/ana.22001. PMID 20373339. "A chronic state of impaired venous drainage from the central nervous system, termed chronic cerebrospinal venous insufficiency (CCSVI), is claimed to be a pathologic phenomenon exclusively seen in multiple sclerosis (MS).".
- ↑ "Embryological background of truncular venous malformation in the extracranial venous pathways as the cause of chronic cerebro spinal venous insufficiency". International Angiology 29 (2): 95–108. April 2010. PMID 20351665. "A similar condition involving the head and neck venous system may cause chronic cerebro-spinal venous insufficiency (CCSVI) and may be involved in the development or exacerbation of multiple sclerosis.".
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 "Venous abnormalities and multiple sclerosis: another breakthrough claim?". The Lancet. Neurology 9 (5): 464–5. May 2010. doi:10.1016/S1474-4422(10)70098-3. PMID 20398855.
- ↑ 6.0 6.1 Ferral, Hector; Lorenz, Jonathan M. (2018-04-13) (in en). Radcases Interventional Radiology. Thieme Medical Publishers. ISBN 978-1-62623-283-9. https://books.google.com/books?id=cH9VDwAAQBAJ&q=CCSVI.
- ↑ 7.0 7.1 7.2 7.3 "Experimental multiple sclerosis vascular shunting procedure halted at Stanford". Annals of Neurology 67 (1): A13-5. January 2010. doi:10.1002/ana.21969. PMID 20186848.
- ↑ 8.0 8.1 8.2 8.3 "Medical device conflict of interest in the CCSVI debate". Annals of Neurology 71 (3): A6-8. March 2012. doi:10.1002/ana.23560. PMID 22451214.
- ↑ 9.0 9.1 FDA (May 2012). "FDA Safety Communication: Chronic Cerebrospinal Venous Insufficiency Treatment in Multiple Sclerosis Patients". https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm303318.htm.
- ↑ "Safety Alerts for Human Medical Products - Balloon angioplasty devices to treat autonomic dysfunction: FDA Safety Communication - FDA concern over experimental procedures" (in en). https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm545619.htm.
- ↑ "Efficacy and Safety of Extracranial Vein Angioplasty in Multiple Sclerosis: A Randomized Clinical Trial". JAMA Neurology 75 (1): 35–43. January 2018. doi:10.1001/jamaneurol.2017.3825. PMID 29150995.
- ↑ "Safety and efficacy of venoplasty in MS: A randomized, double-blind, sham-controlled phase II trial". Neurology 91 (18): e1660–e1668. October 2018. doi:10.1212/WNL.0000000000006423. PMID 30266886.
- ↑ 13.0 13.1 13.2 13.3 13.4 13.5 13.6 "Chronic cerebro-spinal venous insufficiency (CCSVI) and multiple sclerosis". Neurological Sciences 32 (1): 17–21. February 2011. doi:10.1007/s10072-010-0458-3. PMID 21161309.
- ↑ 14.00 14.01 14.02 14.03 14.04 14.05 14.06 14.07 14.08 14.09 14.10 "Chronic cerebrospinal venous insufficiency and the doubtful promise of an endovascular treatment for multiple sclerosis". Journal of NeuroInterventional Surgery 2 (4): 309–11. December 2010. doi:10.1136/jnis.2010.003947. PMID 21990639.
- ↑ 15.0 15.1 "Percutaneous transluminal angioplasty for treatment of chronic cerebrospinal venous insufficiency (CCSVI) in people with multiple sclerosis". The Cochrane Database of Systematic Reviews 5 (7): CD009903. May 2019. doi:10.1002/14651858.CD009903.pub3. PMID 31150100.
- ↑ "Prevalence of extracranial venous narrowing on catheter venography in people with multiple sclerosis, their siblings, and unrelated healthy controls: a blinded, case-control study". Lancet 383 (9912): 138–45. January 2014. doi:10.1016/S0140-6736(13)61747-X. PMID 24119384.
- ↑ "Chronic cerebrospinal venous insufficiency in multiple sclerosis: the final curtain". Lancet 383 (9912): 106–8. January 2014. doi:10.1016/S0140-6736(13)61912-1. PMID 24119383. https://zenodo.org/record/3423610. Retrieved 29 October 2019.
- ↑ "The unsolved puzzle of multiple sclerosis and venous function". Journal of Neurology, Neurosurgery, and Psychiatry 80 (4): 358. April 2009. doi:10.1136/jnnp.2008.168179. PMID 19289474.
- ↑ 19.0 19.1 "Anomalous venous blood flow and iron deposition in multiple sclerosis". Journal of Cerebral Blood Flow and Metabolism 29 (12): 1867–78. December 2009. doi:10.1038/jcbfm.2009.180. PMID 19724286.
- ↑ "The big idea: iron-dependent inflammation in venous disease and proposed parallels in multiple sclerosis". Journal of the Royal Society of Medicine 99 (11): 589–93. November 2006. doi:10.1177/014107680609901122. PMID 17082306. PMC 1633548. http://www.jrsm.org/cgi/pmidlookup?view=long&pmid=17082306.
- ↑ 21.00 21.01 21.02 21.03 21.04 21.05 21.06 21.07 21.08 21.09 21.10 21.11 "Chronic cerebrospinal venous insufficiency and multiple sclerosis". Annals of Neurology 67 (3): 286–90. March 2010. doi:10.1002/ana.22001. PMID 20373339. http://www3.interscience.wiley.com/journal/123283159/abstract.
- ↑ 22.0 22.1 22.2 ""Liberation treatment" for chronic cerebrospinal venous insufficiency in multiple sclerosis: the truth will set you free". Brain and Behavior 5 (1): 3–12. January 2015. doi:10.1002/brb3.297. PMID 25722945.
- ↑ "Is there any relation between chronic cerebrospinal venous insufficiency and multiple sclerosis? - a critical review". Polish Journal of Radiology 79: 131–6. 2014. doi:10.12659/PJR.890379. PMID 24917892.
- ↑ 24.0 24.1 24.2 "Chronic cerebrospinal venous insufficiency and multiple sclerosis: a comprehensive meta-analysis of case-control studies". Therapeutic Advances in Neurological Disorders 7 (2): 114–36. March 2014. doi:10.1177/1756285613499425. PMID 24587827.
- ↑ 25.0 25.1 Jeffrey, Susan (3 December 2009). "CCSVI in Focus at ECTRIMS: New Data but Still Little Clarity". Medscape. http://www.medscape.com/viewarticle/732683.
- ↑ "Embryological background of truncular venous malformation in the extracranial venous pathways as the cause of chronic cerebro spinal venous insufficiency". International Angiology 29 (2): 95–108. April 2010. PMID 20351665. http://www.fondazionehilarescere.org/pdf/03-2518-ANGY.pdf. Retrieved 13 June 2010.
- ↑ "Blood brain barrier compromise with endothelial inflammation may lead to autoimmune loss of myelin during multiple sclerosis". Current Neurovascular Research 6 (2): 132–9. May 2009. doi:10.2174/156720209788185605. PMID 19442163.
- ↑ "Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis". Neurology 74 (13): 1041–7. March 2010. doi:10.1212/WNL.0b013e3181d6b125. PMID 20350978.
- ↑ "Chronic cerebrospinal venous insufficiency and multiple sclerosis". Annals of Neurology 68 (2): 270. August 2010. doi:10.1002/ana.22067. PMID 20695021.
- ↑ Primary studies during 2010 on neuroimaging, CCSVI, and MS:
- "Normal CSF ferritin levels in MS suggest against etiologic role of chronic venous insufficiency". Neurology 75 (18): 1617–22. November 2010. doi:10.1212/WNL.0b013e3181fb449e. PMID 20881272.
- "Iron stores and cerebral veins in MS studied by susceptibility weighted imaging". International Angiology 29 (2): 149–57. April 2010. PMID 20351671.
- ↑ "The controversy of CCSVI and iron in multiple sclerosis: is ferritin the key?". Neurology 75 (18): 1581–2. November 2010. doi:10.1212/WNL.0b013e3181fb44f0. PMID 20881276.
- ↑ "Custom CGH array profiling of copy number variations (CNVs) on chromosome 6p21.32 (HLA locus) in patients with venous malformations associated with multiple sclerosis". BMC Medical Genetics 11: 64. April 2010. doi:10.1186/1471-2350-11-64. PMID 20426824. (primary source)
- ↑ "Chronic cerebrospinal vascular insufficiency is not associated with HLA DRB1*1501 status in multiple sclerosis patients". PLOS ONE 6 (2): e16802. February 2011. doi:10.1371/journal.pone.0016802. PMID 21340025. Bibcode: 2011PLoSO...616802W. (primary source)
- ↑ "Extracranial Doppler sonographic criteria of chronic cerebrospinal venous insufficiency in the patients with multiple sclerosis". International Angiology 29 (2): 109–14. April 2010. PMID 20351666.
- ↑ "Use of neck magnetic resonance venography, Doppler sonography and selective venography for diagnosis of chronic cerebrospinal venous insufficiency: a pilot study in multiple sclerosis patients and healthy controls". International Angiology 29 (2): 127–39. April 2010. PMID 20351669.
- ↑ 36.0 36.1 36.2 36.3 36.4 36.5 36.6 36.7 "What went wrong? The flawed concept of cerebrospinal venous insufficiency". Journal of Cerebral Blood Flow and Metabolism 33 (5): 657–68. May 2013. doi:10.1038/jcbfm.2013.31. PMID 23443168.
- ↑ Jeffrey, Susan (3 December 2009). "Endovascular Treatment of Cerebrospinal Venous Insufficiency Safe, May Provide Benefit in MS". Medscape. http://www.medscape.com/viewarticle/713367.
- ↑ 38.0 38.1 "Cardiovascular and Interventional Radiological Society of Europe commentary on the treatment of chronic cerebrospinal venous insufficiency". CardioVascular and Interventional Radiology 34 (1): 1–2. February 2011. doi:10.1007/s00270-010-0050-5. PMID 21136256.
- ↑ "Interventional endovascular management of chronic cerebrospinal venous insufficiency in patients with multiple sclerosis: a position statement by the Society of Interventional Radiology, endorsed by the Canadian Interventional Radiology Association". Journal of Vascular and Interventional Radiology 21 (9): 1335–7. September 2010. doi:10.1016/j.jvir.2010.07.004. PMID 20800776.
- ↑ 40.0 40.1 40.2 "Study casts doubt on MS treatment". BootsWebMD. 2013-03-18. http://www.webmd.boots.com/news/20130318/doubt-on-ccsvi-ms-treatment.
- ↑ Favaro, Avis (9 April 2010). "Kuwait to offer controversial MS treatment". CTV Winnipeg. https://www.ctvnews.ca/kuwait-to-offer-controversial-ms-treatment-1.500683.
- ↑ Melnychuk, Phil (16 September 2010). "Seeking liberation". Bclocalnews.com. http://www.bclocalnews.com/tri_city_maple_ridge/mapleridgenews/community/103094069.html/.
- ↑ "A prospective open-label study of endovascular treatment of chronic cerebrospinal venous insufficiency". Journal of Vascular Surgery 50 (6): 1348–58.e1–3. December 2009. doi:10.1016/j.jvs.2009.07.096. PMID 19958985. http://www.jvascsurg.org/article/S0741521409015687/pdf.
- ↑ Laino, Charlene (17 June 2010). "New Theory on CCSVI and MS Needs Further Study, Experts Say". Neurology Today. http://www.aan.com/elibrary/neurologytoday/?event=home.showArticle&id=ovid.com:/bib/ovftdb/00132985-201006170-00013.
- ↑ "Endovascular treatment for chronic cerebrospinal venous insufficiency: is the procedure safe?". Phlebology 25 (6): 286–95. December 2010. doi:10.1258/phleb.2010.010053. PMID 21107001.
- ↑ Agrell, Siri (29 April 2010). "Legal fears thwart doctor's bid for 'liberation' from MS pain". The Globe and Mail (Toronto). https://www.theglobeandmail.com/news/national/legal-fears-thwart-doctors-bid-for-liberation-from-ms-pain/article1550377/.
- ↑ "Multiple sclerosis pathology: evolution of pathogenetic concepts". Brain Pathology 15 (3): 217–22. July 2005. doi:10.1111/j.1750-3639.2005.tb00523.x. PMID 16196388.[verification needed]
- ↑ "Encephalitis and sclerotic plaques produced by venular obstruction". Arch Neurol Psychiatry 33 (5): 929–940. 1935. doi:10.1001/archneurpsyc.1935.02250170015002.
- ↑ 49.00 49.01 49.02 49.03 49.04 49.05 49.06 49.07 49.08 49.09 49.10 49.11 49.12 49.13 49.14 49.15 49.16 49.17 49.18 49.19 49.20 49.21 "Media, politics and science policy: MS and evidence from the CCSVI Trenches". BMC Medical Ethics 14: 6. February 2013. doi:10.1186/1472-6939-14-6. PMID 23402260.
- ↑ 50.0 50.1 50.2 50.3 50.4 50.5 Sinemma, Jodie (10 August 2010). "MS therapy divides even sufferers, as social media drives hype". Calgary Herald. https://calgaryherald.com/health/therapy+divides+even+sufferers+social+media+drives+hype/3383016/story.html.[yes|permanent dead link|dead link}}]
- ↑ 51.0 51.1 51.2 51.3 Locke, Tim (12 October 2010). "CCSVI for MS in the UK". WebMD Health News (WebMD UK Limited and Boots UK Limited). http://www.webmd.boots.com/news/20101012/ccsvi-for-ms-in-the-uk?print=true.
- ↑ 52.0 52.1 "Information Sheet on Multiple Sclerosis (MS) and "Chronic Cerebrospinal Venous Insufficiency" (CCSVI)". Alberta Health Services. 6 August 2010. http://www.albertahealthservices.ca/feat/ne-feat-ccsvi-ms-info-sheet.pdf.
- ↑ Cowan, Pamela (6 August 2010). "'Placebo effect' a concern with controversial MS treatment: Experts". https://ottawacitizen.com/health/Placebo+effect+concern+with+controversial+treatment+Experts/3367299/story.html?cid=megadrop_story.[yes|permanent dead link|dead link}}]
- ↑ "MS group to fund research into 'liberation procedure'". The Globe and Mail (Toronto). 23 November 2009. https://www.theglobeandmail.com/news/national/ms-group-to-fund-research-into-liberation-procedure/article1374954/.
- ↑ "MS Society's stand sparks resignation". The Telegram. 1 September 2010. http://www.thetelegram.com/News/Local/2010-09-01/article-1716828/MS-Society%26rsquo%3Bs-stand-sparks-resignation/1.
- ↑ "MS Society sets aside $1M in case CCSVI patient trial developed and approved". CBC News. 16 September 2010. http://www.cbc.ca/cp/health/TG3929.html. [|permanent dead link|dead link}}]
- ↑ "Premiers jump the gun on MS treatment". Calgary Herald. 4 August 2010. https://calgaryherald.com/life/Premiers+jump+treatment/3356742/story.html.[yes|permanent dead link|dead link}}]
- ↑ "Premiers to debate MS treatment". CBC News. 2 August 2010. http://www.cbc.ca/health/story/2010/08/02/pei-ms-treatment-584.html.
- ↑ "MS therapy trial urged by Manitoba minister". CBC News. Canadian Press. 16 August 2010. https://www.cbc.ca/news/canada/manitoba/ms-therapy-trial-urged-by-manitoba-minister-1.904451.
- ↑ "CIHR makes recommendations on Canadian MS research priorities". Canadian Institutes of Health Research. 31 August 2010. http://www.cihr-irsc.gc.ca/e/42382.html.
- ↑ "Health minister rejects MS therapy trial". CBC News. 2 September 2010. http://www.cbc.ca/canada/newfoundland-labrador/story/2010/09/01/ms-ccsvi-liberation-aglukkaq.html.
- ↑ Weeks, Carly (29 June 2011). "Ottawa to fund clinical trials for controversial MS treatment". The Globe and Mail. http://www.cbc.ca/canada/newfoundland-labrador/story/2010/09/01/ms-ccsvi-liberation-aglukkaq.html.
- ↑ 63.0 63.1 ""I knew what was going to happen if I did nothing and so I was going to do something": faith, hope, and trust in the decisions of Canadians with multiple sclerosis to seek unproven interventions abroad". BMC Health Services Research 14: 445. September 2014. doi:10.1186/1472-6963-14-445. PMID 25265935.
- ↑ 64.0 64.1 64.2 64.3 "Navigating the "liberation procedure": a qualitative study of motivating and hesitating factors among people with multiple sclerosis". Patient Preference and Adherence 8: 1205–13. 2014. doi:10.2147/PPA.S65483. PMID 25228799.
- ↑ "Evidence against the involvement of chronic cerebrospinal venous abnormalities in multiple sclerosis. A case-control study". PLOS ONE 8 (8): e72495. 14 August 2013. doi:10.1371/journal.pone.0072495. PMID 23967312. Bibcode: 2013PLoSO...872495R.
- ↑ "Prevalence of extracranial venous narrowing on catheter venography in people with multiple sclerosis, their siblings, and unrelated healthy controls: a blinded, case-control study". Lancet 383 (9912): 138–45. January 2014. doi:10.1016/s0140-6736(13)61747-x. PMID 24119384. http://www.thelancet.com/journals/lancet/issue/vol383no9912/PIIS0140-6736(14)X6065-4.
- ↑ Definitive imaging study finds no link between venous narrowing and multiple sclerosis, University of British Columbia, media release 8 October 2013.
- ↑ "'Scientific quackery': UBC study says it's debunked controversial MS procedure". CBC News. 2017-03-08. http://www.cbc.ca/news/health/multiple-sclerosis-liberation-therapy-clinical-trial-1.4014494.
- ↑ "What's the Latest on CCSVI and MS? Discover New Research at the National CCSVI Society's Major Canadian CCSVI Forum". Reuters. 6 July 2011. https://www.reuters.com/article/2011/07/06/idUS119820+06-Jul-2011+MW20110706.
- ↑ "UIP self report". http://www.uip-phlebology.org/?page_id=2.
- ↑ "Italian group offers $4.5M to fund new MS research". CTV News. 27 January 2010. http://www.ctvnews.ca/italian-group-offers-4-5m-to-fund-new-ms-research-1.478128.
- ↑ "Diagnosis and treatment of venous malformations. Consensus document of the International Union of Phlebology (IUP)-2009". International Angiology 28 (6): 434–51. December 2009. PMID 20087280.
- ↑ "CCSVI – current studies". mssociety.org.uk. http://www.mssociety.org.uk/research/az_of_ms_research/cd/ccsvi_studies.html.
- ↑ "Recommendations for multimodal noninvasive and invasive screening for detection of extracranial venous abnormalities indicative of chronic cerebrospinal venous insufficiency: a position statement of the International Society for Neurovascular Disease". Journal of Vascular and Interventional Radiology 25 (11): 1785–94.e17. November 2014. doi:10.1016/j.jvir.2014.07.024. PMID 25255703.
Further reading
- "A Controversial 'Cure' for M.S.". The New York Times. 26 October 2012. https://www.nytimes.com/2012/10/28/magazine/a-controversial-cure-for-multiple-sclerosis.html. Overview, interviews with proponents and critics.
- CCSVI as the Cause of Multiple Sclerosis: The Science Behind the Controversial Theory. McFarland Health Topics. 10 January 2014. ISBN 978-0-7864-8628-1.
External links
| Classification |
|---|
 |