Medicine:Hidradenitis suppurativa
| Hidradenitis suppurativa | |
|---|---|
| Other names | Acne inversa, apocrine acne, Verneuil's disease, Velpeau's disease[1] |
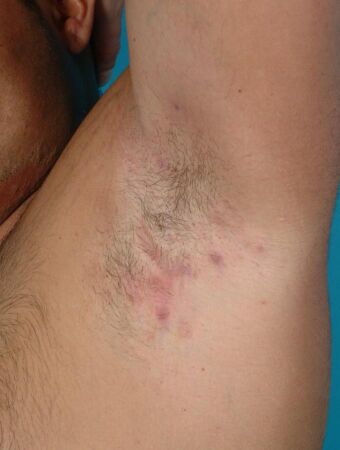 | |
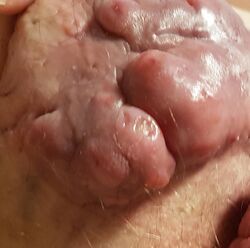
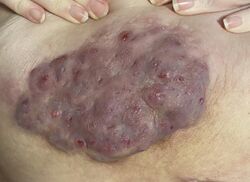
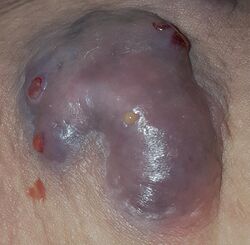
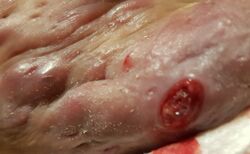
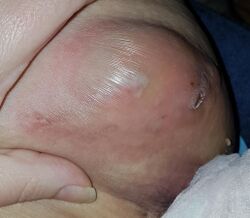
| Hidradenitis suppurativa (Hurley's stage II) in the left armpit | |
| Specialty | Dermatology |
| Symptoms | Multiple inflamed and swollen skin lesions[2] |
| Usual onset | Childhood and Young adulthood[2] |
| Duration | Long-term[2] |
| Types | Stage I, II, III[1] |
| Causes | Unknown[3] |
| Diagnostic method | Based on symptoms[2] |
| Differential diagnosis | Acne, acne conglobata, pilonidal cysts[2] |
| Treatment | Warm baths, laser therapy, surgery[2][4] |
| Medication | Secukinumab, antibiotics, immunosuppressive medication[2] |
| Frequency | 1–4% of people, when mild cases are included[2][3] |
| Deaths | Rare[1] |
Hidradenitis suppurativa (HS), sometimes known as acne inversa or Verneuil's disease, is a long-term dermatological condition characterized by the occurrence of inflamed and swollen lumps.[2][3] These are typically painful and break open, releasing fluid or pus.[3] The areas most commonly affected are the underarms, under the breasts, perineum, buttocks, and the groin.[1] Scar tissue remains after healing.[1] HS may significantly limit many everyday activities, for instance, walking, hugging, moving, and sitting down. Sitting disability may occur in patients with lesions in the sacral, gluteal, perineal, femoral, groin or genital regions. Prolonged periods of sitting down can also worsen the condition of the skin of these patients.[5][6][7][8][9]
The exact cause is usually unclear but believed to involve a combination of genetic and environmental factors.[3] About a third of people with the disease have an affected family member.[3] Other risk factors include obesity and smoking.[3] The condition is not caused by an infection, poor hygiene, or the use of deodorant.[3][4] Instead, it is believed to be caused by hair follicles being obstructed,[10][1] with the nearby apocrine sweat glands being strongly implicated in this obstruction.[1][11] The sweat glands may or may not be inflamed.[1] Diagnosis is based on the symptoms.[2]
No cure is known,[4] though surgical excision with wet-to-dry dressings, proper wound care, and warm baths or showering with a pulse-jet shower may be used in those with mild disease.[4] Cutting open the lesions to allow them to drain does not result in significant benefit.[2] While antibiotics are commonly used, evidence for their use is poor.[4] Immunosuppressive medication may also be tried.[2] In those with more severe disease, laser therapy or surgery to remove the affected skin may be viable.[2] Rarely, a skin lesion may develop into skin cancer.[3]
If mild cases of HS are included, then the estimate of its frequency is from 1–4% of the population.[2][3] Women are three times more likely to be diagnosed with it than men.[2] Onset is typically in young adulthood and may become less common after 50 years old.[2] It was first described between 1833 and 1839 by French anatomist Alfred Velpeau.[1][12]
Terminology
Although hidradenitis suppurativa is often called acne inversa, it is not a form of acne. Hidradenitis suppurativa lacks the core defining features of acne, such as the presence of closed comedones and increased sebum production.[13]
Causes
The exact cause of hidradenitis suppurativa remains unknown,[11][14] and there has, in the recent past, been notable disagreement among experts in this regard.[15] The condition, however, likely stems from both genetic and environmental causes.[3] Specifically, an immune-mediated pathology has been proposed,[11] although environmental factors have not been ruled out.[3]
Lesions will occur in any body area with hair follicles,[15] and/or sweat glands.[16] Areas such as the axilla, groin, and perineal region are more commonly involved. This theory includes most of these potential indicators:[17]
- Post-pubescent individuals[18]
- Blocked hair follicles or blocked apocrine sweat glands
- Excessive sweating
- Androgen dysfunction
- Genetic disorders that alter cell structure
The historical understanding of the disease suggests dysfunctional apocrine glands[19] or dysfunctional hair follicles,[20] possibly triggered by a blocked gland, which creates inflammation, pain, and a swollen lesion.
Triggering factors
Several triggering factors should be taken into consideration:
- Obesity[21] is an exacerbating rather than a triggering factor,[22] through mechanical irritation, occlusion, and skin maceration.
- Tight clothing,[21] and clothing made of heavy, nonbreathable materials[23]
- Deodorants, depilation products, shaving of the affected area – their association with HS is still an ongoing debate among researchers.[24]
- Drugs, in particular oral contraceptive pills,[25] cigarette smoking,[26][27] and lithium.[28]
- Hot and especially humid climates.[29]
- Stress[27]
Predisposing factors
- Genetic factors:[22] an autosomal dominant inheritance pattern has been proposed.[30]
- Endocrine factors:
- Sex hormones, especially an excess of androgens, are thought to be involved, although the apocrine glands are not sensitive to these hormones.[31] Women often have outbreaks before their menstrual period and after pregnancy; HS severity usually decreases during pregnancy and after menopause.
- Diabetes mellitus is common in hidradenitis suppurativa and seems to be a risk factor.[32]
Some cases have been found to result from mutations in the NCSTN, PSEN1, or PSENEN genes. The genes produce proteins that are all components of a complex called gamma- (γ-) secretase. This complex cuts apart (cleaves) many different proteins, which is a crucial step in several chemical signaling pathways. One of these pathways, known as notch signaling, is essential for the normal maturation and division of hair follicle cells and other types of skin cells. Notch signaling influences normal immune system function. Studies suggest mutations in the NCSTN, PSEN1, or PSENEN genes impair notch signaling in hair follicles. Although little is known about the mechanism, abnormal notch signaling appears to promote the development of nodules and lead to skin inflammation.[33] In addition, the composition of the intestinal microflora and as a consequence dietary patterns appear to play a role. Although dysbiosis of the cutaneous microbiome apparent in HS is not observed, the concurrent existence of inflammatory gut and skin diseases has led to the hypothesis of a gut-skin axis in which gut microbiota is implicated. Indeed, analysis of bacterial taxa in fecal samples from HS patients supports the possibility of a role for intestinal microbial alterations in this chronic inflammatory skin disease.[34]
Precocious puberty is more common among children and adolescents with hidradenitis suppurativa (HS) compared to those without the condition, according to a recent case-control study.[35]
This article relies too much on references to primary sources. (April 2025) (Learn how and when to remove this template message) |
An analysis of the Explorys database revealed that pediatric patients with precocious puberty have double the risk of developing HS, even after adjusting for factors like demographic characteristics and body mass index (BMI).[36]
Diagnosis
Early diagnosis is essential in avoiding tissue damage. However, HS is often misdiagnosed or diagnosed late due to healthcare professionals not being aware of the condition or people not consulting with a physician.[37][38] Globally, the diagnosis is delayed more than 7 years in average after symptoms appear. This is much longer than with other skin conditions.[39]
Stages
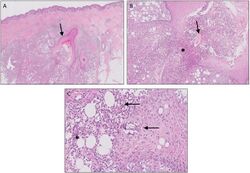
Hidradenitis suppurativa presents itself in three stages.[19][41] Due to the large spectrum of clinical severity and the severe impact on quality of life, a reliable method for evaluating HS severity is needed.
Hurley's staging system
Hurley's staging system was the first classification system proposed and is still in use for the classification of patients with skin diseases (i.e., psoriasis, HS, acne). Hurley separated patients into three groups based largely on the presence and extent of cicatrization and sinuses. It has been used as a basis for clinical trials in the past and to determine appropriate therapy for patients. These three stages are based on Hurley's staging system, which relies on the subjective extent of the diseased tissue. Hurley's three stages of hidradenitis suppurativa are:[42]
| Stage | Characteristics |
|---|---|
| I | Solitary or multiple isolated abscess formation without scarring or sinus tracts (A few minor sites with rare inflammation; may be mistaken for acne.) |
| II | Recurrent abscesses, single or multiple widely separated lesions, with sinus tract formation (Frequent inflammation restricts movement and may require minor surgery such as incision and drainage.) |
| III | Diffuse or broad involvement across a regional area with multiple interconnected sinus tracts and abscesses (Inflammation of sites to the size of golf balls, or sometimes baseballs; scarring develops, including subcutaneous tracts of infection – see fistula. Obviously, patients at this stage may be unable to function.) |
Sartorius staging system
The Sartorius staging system is more sophisticated than Hurley's. Sartorius et al. suggested that the Hurley system is not sophisticated enough to assess treatment effects in clinical trials during research. This classification allows for better dynamic monitoring of the disease severity in individual patients. The elements of this staging system are:[43]
- Anatomic regions involved (axilla, groin, gluteal, or other region or inframammary region left or right)
- Number and types of lesions involved (abscesses, nodules, fistulas or sinuses, scars, points for lesions of all regions involved)
- The distance between lesions, in particular, the longest distance between two relevant lesions (i.e., nodules and fistulas in each region or size if only one lesion is present)
- The presence of normal skin in between lesions (i.e., if all lesions are separated by normal skin)
Points are accumulated in the above categories and added to give a regional and total score. In addition, the authors recommend adding a visual analog scale for pain or using the dermatology life quality index (DLQI, or the 'skindex') when assessing HS.[44]
Treatment
Treatment depends upon the presentation and severity of the disease. Due to the poorly studied nature of the disease, the effectiveness of medications and therapies was unclear.[45] Clear and sensitive communication from health care professionals, and social and psychological interventions can help manage the emotional impact of the condition and aid necessary lifestyle changes.[37][38] In May 2023, the European Commission (EC) approved Cosentyx (secukinumab) for active moderate to severe hidradenitis suppurativa in adults.[46]
Other possible treatments include the following:
Cryotherapy
Cryotherapy has demonstrated efficacy against the disease, with 88% of persistent lesions resolving in a clinical trial of 23 patients.[47]
Lifestyle
Warm baths may be tried in those with mild disease.[4] Weight loss and smoking cessation are recommended.[2]
Medication
- Antibiotics: taken by mouth, these are used for their anti-inflammatory properties rather than to treat infection. Most effective is a combination of rifampicin and clindamycin given concurrently for 2–3 months. Popular antibiotics also include tetracycline and minocycline.[48] Topical clindamycin has been shown to have an effect in double-blind placebo-controlled studies.[49] In a retrospective review and telephone survey, intravenous ertapenem therapy showed clinical improvement with 80.3% of subjects reporting medium to high satisfaction and 90.8% would recommend ertapenem to other patients.[50]
- Corticosteroid injections, also known as intralesional steroids, can be useful for localized disease if the drug can be prevented from escaping via the sinuses.
- Antiandrogen therapy, hormonal therapy with antiandrogenic medications such as spironolactone, flutamide, cyproterone acetate, ethinylestradiol, finasteride, dutasteride, and metformin, are effective in clinical studies.[51][52][53] However, the quality of available evidence is low and does not presently allow for robust evidence-based recommendations.[51][52]
- Intravenous infusion or subcutaneous injection of anti-inflammatory (TNF inhibitors; anti-TNF-alpha) drugs such as infliximab, and etanercept[54] This use of these drugs is not currently Food and Drug Administration (FDA) approved and is somewhat controversial, so may not be covered by insurance.
- Biologics: Various biologics can improve HS lesions.[55] Specifically adalimumab at weekly intervals is useful.[56] Adalimumab and Secukinumab[57][58] are both approved by the FDA for the treatment of HS as of 2023.
- Topical isotretinoin is usually ineffective in people with HS, and is more commonly known as a medication for the treatment of acne vulgaris. Individuals affected by HS who responded to isotretinoin treatment tended to have milder cases of the condition.[59]
- Zinc and Nicotinamide, at doses of 90 mg and 30 mg respectively, have shown efficacy against mild to moderate hidradenitis suppurativa in a controlled retrospective clinical trial.[60]
Surgery
When the process becomes chronic, wide surgical excision is the procedure of choice.
Wounds in the affected area do not heal by secondary intention, and immediate or delayed application of a split-thickness skin graft is an option.[17] Another option is covering the defect with a perforator flap. With this technique, the (mostly totally excised) defect is covered with tissue from an area nearby. For example, the axilla with a fully excised defect of 15 × 7 cm can be covered with a thoracodorsal artery perforator flap. . A less invasive excision procedure called Skin-Tissue-sparing Excision with Electrosurgical Peeling or "STEEP" has also been developed for treating moderate to severe disease.[61]
Laser hair removal
The 1064-nm wavelength laser for hair removal may aid in the treatment of HS.[62] A randomized control study has shown improvement in HS lesions with the use of a Physics:Nd:YAG laser.[63]
Botox injection
A 2022 study reported that administration of Botulinum toxin resulted in either clinical improvement or improved quality of life in 96.8% (n = 30/31) of HS patients. The level of evidence was moderate. It concluded that the treatment was a safe and potentially effective alternative for hidradenitis suppurativa patients resistant to standard-of-care therapies.[64]
Prognosis
In stage III disease, as classified by Hurley's staging system, fistulae left undiscovered, undiagnosed, or untreated, can rarely lead to the development of squamous cell carcinoma in the anus or other affected areas.[65][66] Other stage III chronic sequelae may also include anemia, multi-localized infections, amyloidosis, and arthropathy. Stage III complications have been known to lead to sepsis, but clinical data are still uncertain.
Comorbidities and complications
Endocrine diseases are more common in people who have HS.[67] Diabetes mellitus may be both a causal factor contributing to the evolution and/or severity of HS and a consequence of inflammation in HS.[32][68] Thyroid disorders are more common, too, in HS.[69]
- Contractures and reduced mobility of the lower limbs and axillae due to fibrosis and scarring occur. Severe lymphedema may develop in the lower limbs.
- Local and systemic infections (meningitis, bronchitis, pneumonia, etc.), are seen, which may even progress to sepsis.
- Higher risk for diabetes mellitus, high blood pressure, heart attacks, and metabolic syndrome.[32] Persons affected by HS have reduced insulin sensitivity (SPINA-GR), which, if uncompensated by increased pancreatic beta cell function, can result in prediabetes and overt diabetes mellitus.[70] Treatment of HS may contribute to remission of diabetes.[68]
- HS is associated with an increased risk of having polycystic ovarian syndrome.[71][72]
- Interstitial keratitis
- Pyoderma gangrenosum[72]
- Pilonidal disease[72]
- Dyslipidemia[72]
- Anal, rectal, or urethral fistulae[73]
- Normochromic or hypochromic anemia[74]
- People with HS may be at increased risk for autoimmune disorders, including ankylosing spondylitis, rheumatoid arthritis, psoriatic arthritis, and inflammatory bowel diseases, such as Crohn's disease.[72][75]
- Squamous cell carcinoma has been found on rare occasions in chronic hidradenitis suppurativa of the anogenital region.[76] The mean time to the onset of this type of lesion is 10 years or more and the tumors are usually highly aggressive.
- Tumors of the lung and oral cavity, and liver cancer[77]
- Hypoproteinemia and amyloidosis, which can lead to kidney failure and death[78]
- Seronegative and usually asymmetric arthropathy: pauciarticular arthritis, polyarthritis/polyarthralgia syndrome.[79]
Impact on mental health
HS is a painful and socially isolating condition that leads to a negative impact on mental health as well. A 2020 meta-analysis found that 21% of people with HS have depression, including major depression and 12% have anxiety, including generalized anxiety disorder and a higher risk of suicide.[72][80] A 2020 study found that people with HS have suicide rates more than double the rates in controls, and also have a higher risk of attempting suicide.[81]
Epidemiology
Prevalence
Estimates of the prevalence of HS vary worldwide, and there is no accepted generalization. In the US, the prevalence is estimated to be 0.1% while in Europe it is thought to be 1% or more.[81]
Biological Sex
In North America and Europe, women are three times more likely to have HS. However, in South Korea, men are twice as likely to have HS.[81]
Age
HS is the most prevalent in people in their 40s and 50s.[81]
History
- From 1833 to 1839, in a series of three publications, Velpeau identified and described a disease now known as hidradenitis suppurativa.[82]
- In 1854, Verneuil described hidradenitis suppurativa as hidrosadénite Phlegmoneuse. This is how HS obtained its alternate name "Verneuil's disease".[83]
- In 1922, Schiefferdecker hypothesized a pathogenic link between "acne inversa" and human sweat glands.[84]
- In 1956, Pillsbury et al.[85] coined the term follicular occlusion triad for the common association of hidradenitis suppurativa, acne conglobata and dissecting cellulitis of the scalp. Modern clinical research still employs Pillsbury's terminology for these conditions' descriptions.[86]
- In 1975, Plewig and Kligman, following Pillsbury's research path, modified the "acne triad", replacing it with the "acne tetrad: acne triad, plus pilonidal sinus".[87] Plewig and Kligman's research follows in Pillsbury's footsteps, offering explanations of the symptoms associated with hidradenitis suppurativa.
- In 1989, Plewig and Steger's research led them to rename hidradenitis suppurativa, calling it "acne inversa" – which is not still used today in medical terminology, although some individuals still use this outdated term.[88]
A surgeon from Paris, Velpeau, described an unusual inflammatory process with the formation of superficial axillary, submammary, and perianal abscesses, in a series of three publications from 1833 to 1839. One of his colleagues, also located in Paris, named Verneuil, coined the term hidrosadénite phlegmoneuse about 15 years later. This name for the disease reflects the former pathogenetic model of acne inversa, which is considered inflammation of sweat glands as the primary cause of hidradenitis suppurativa. In 1922, Schiefferdecker suspected a pathogenic association between acne inversa and apocrine sweat glands. In 1956, Pillsbury postulated follicular occlusion as the cause of acne inversa, which they grouped together with acne conglobata and perifolliculitis capitis abscendens et suffodiens ("dissecting cellulitis of the scalp") as the "acne triad". Plewig and Kligman added another element to their acne triad, pilonidal sinus. Plewig et al. noted that this new "acne tetrad" includes all the elements found in the original "acne triad", in addition to a fourth element, pilonidal sinus. In 1989, Plewig and Steger introduced the term "acne inversa", indicating a follicular source of the disease and replacing older terms such as "Verneuil disease".
| Author | Year | Findings |
|---|---|---|
| Velpeau | 1839 | First description of the hidradenitis suppurativa |
| Verneuil | 1854 | "Hidrosadénite phlegmoneuse" |
| Pillsbury | 1956 | Acne triad (hidradenitis suppurativa, perifolliculitis capitis abscendens et suffodiens, acne congoblata) |
| Plewig & Kligman | 1975 | Acne tetrad (acne triad + pilonidal sinus) |
| Plewig & Steger | 1989 | Acne inversa |
Other names
Hidradenitis suppurativa has been referred to by multiple names in the literature and various cultures. Some of these are also used to describe different diseases or specific instances of this disease.[41]
- Acne conglobata – not really a synonym – this is a similar process but in classic acne areas of the chest and back
- Acne inversa – a proposed new term[89][90] which has not gained widespread favor.[91]
- Apocrine acne – an outdated term based on the disproven concept that apocrine glands are primarily involved, though many do have apocrine gland infection
- Apocrinitis – another outdated term based on the same thesis
- Fox-den disease – a term not used in medical literature, based on the deep fox den–like sinuses
- Hidradenitis supportiva – a misspelling
- Pyodermia fistulans significa – now considered archaic
- Verneuil's disease – recognizing the surgeon whose name is most often associated with the disorder as a result of his 1854–1865 studies[92]
Histology
| Author | Year | Major Features |
|---|---|---|
| Plewig & Steger[88] | 1989 | Initial hyperkeratosis of the follicular infundibulum. Bacterial super-infection and follicle rupture. Granulomatous inflammatory reaction of the connective tissue. Apocrine and eccrine sweat glands secondarily involved. |
| Yu & Cook[93] | 1990 | Cysts and sinus tracts lined with epithelium, in part with hair shafts. Inflammation of apocrine sweat glands only if eccrine sweat glands and hair follicles are also inflamed. |
| Boer & Weltevreden[94] | 1996 | Primary inflammation of the follicular infundibulum. Apocrine sweat glands are secondarily involved. |
Society and culture
Experiences of people with HS
HS can have a strong negative impact on people's lives, as well as physical and mental health. People with HS often feel stigmatized and embarrassed by their condition. Many try to hide the symptoms, which can lead to impaired relationships and social isolation. A multidisciplinary approach by healthcare professionals, social support networks, and psychological interventions can contribute to a better quality of life.[37][38] Compared to other skin diseases, HS has one of the highest Dermatology Life Quality Index (DLQI) scores.[95]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 "Hidradenitis Suppurativa". 2012. https://rarediseases.org/rare-diseases/hidradenitis-suppurativa/.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 "Clinical practice. Hidradenitis suppurativa". The New England Journal of Medicine 366 (2): 158–64. January 2012. doi:10.1056/NEJMcp1014163. PMID 22236226.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 "Hidradenitis suppurativa" (in en). December 2013. https://medlineplus.gov/genetics/condition/hidradenitis-suppurativa/.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 "Hidradenitis suppurativa" (in en). 2017. https://rarediseases.info.nih.gov/diseases/6658/hidradenitis-suppurativa.
- ↑ "Intergluteal contour deformity in hidradenitis suppurativa". https://www.ingentaconnect.com/contentone/mjl/adv/2019/00000099/f0020001/art00024?crawler=true&mimetype=application/pdf.
- ↑ Nazzaro, Gianluca; Passoni, Emanuela; Calzari, Paolo; Barbareschi, Mauro; Muratori, Simona; Veraldi, Stefano; Marzano, Angelo Valerio (2019). "Color Doppler as a tool for correlating vascularization and pain in hidradenitis suppurativa lesions". Skin Research and Technology 25 (6): 830–834. doi:10.1111/srt.12729. PMID 31140660. https://onlinelibrary.wiley.com/doi/pdf/10.1111/srt.12729. Retrieved 18 May 2021.
- ↑ Loh, Tiffany Y.; Hendricks, Aleksi J.; Hsiao, Jennifer L.; Shi, Vivian Yan (2021). "Undergarment and fabric selection in the management of hidradenitis suppurativa". Dermatology 237 (1): 119–124. doi:10.1159/000501611. PMID 31466052. https://www.karger.com/Article/FullText/501611. Retrieved 18 May 2021.
- ↑ Jemec, G.B.E.; Heidenheim, M.; Nielsen, N.H. (1996). "Hidradenitis suppurativa-characteristics and consequences". Clinical and Experimental Dermatology 21 (6): 419–423. doi:10.1111/j.1365-2230.1996.tb00145.x. PMID 9167336. https://onlinelibrary.wiley.com/doi/pdf/10.1111/j.1365-2230.1996.tb00145.x. Retrieved 18 May 2021.
- ↑ "Psychosocial impact of hidradenitis suppurativa: a qualitative study". https://www.ingentaconnect.com/contentone/mjl/adv/2011/00000091/00000003/art00014?crawler=true&mimetype=application/pdf.
- ↑ See section "Genetic Changes"[3]
- ↑ 11.0 11.1 11.2 Pathophysiology of hidradenitis suppurativa (Seminal paper, SCMS Journal); NIH, National Library of Medicine, NCBI; 2017 Jun, 36(2):47–54.
- ↑ Jemec, Gregor; Revuz, Jean; Leyden, James J. (2006) (in en). Hidradenitis Suppurativa. Springer Science & Business Media. p. 5. ISBN 978-3-540-33101-8. https://books.google.com/books?id=hpKFsXwcKlgC&pg=PA5.
- ↑ "Hidradenitis suppurrativa (acne inversa) as a systemic disease". Clinics in Dermatology 32 (3): 397–408. May–June 2014. doi:10.1016/j.clindermatol.2013.11.006. PMID 24767187.
- ↑ See section "Genetic Changes"[3]
- ↑ 15.0 15.1 Medline Plus (2012). "Hidradenitis suppurativa". U.S. National Library of Medicine. https://www.medlineplus.gov/hidradenitissuppurativa.html.
- ↑ Hopkins Medicine Staff (2024). "Inflamed/Infected Sweat Glands (Hidradenitis)". Johns Hopkins Medicine. https://www.hopkinsmedicine.org/health/conditions-and-diseases/hidradenitis-suppurativa.
- ↑ 17.0 17.1 Schawartz's Principles of Surgery, 8th edition, self-assessment, and board review, chapter 15, the skin and subcutaneous tissue, question 16
- ↑ Mayo Clinic Staff (2012). "Definition". Mayo Clinic. http://www.mayoclinic.com/health/hidradenitis-suppurativa/DS00818.
- ↑ 19.0 19.1 DermNet acne/hidradenitis-suppurativa
- ↑ "HSF – What is Hidradenitis Suppurativa? What is HS?". http://www.hs-foundation.org/abouths/what.htm.
- ↑ 21.0 21.1 "Hidradenitis suppurativa: pathogenesis and management". British Journal of Plastic Surgery 56 (5): 451–61. July 2003. doi:10.1016/S0007-1226(03)00177-2. PMID 12890458.
- ↑ 22.0 22.1 Jemec GBE. Body weight in hidradenitis suppurativa. In: Marks R, Plewig G, editors. Acne and Related Disorders. London: Martin Dunitz; 1989. pp. 375–6.
- ↑ "Cigarette smoking as a triggering factor of hidradenitis suppurativa". Dermatology 198 (3): 261–4. 1999. doi:10.1159/000018126. PMID 10393449.
- ↑ "The distribution, size and density of the apocrine glands in hidradenitis suppurativa". The British Journal of Surgery 66 (12): 853–6. December 1979. doi:10.1002/bjs.1800661206. PMID 509057.
- ↑ "Hidradenitis suppurativa associated with use of oral contraceptives". BMJ 298 (6665): 28–9. January 1989. doi:10.1136/bmj.298.6665.28. PMID 2492847.
- ↑ Acharya, P; Mathur, M (April 2020). "Hidradenitis suppurativa and smoking: A systematic review and meta-analysis.". Journal of the American Academy of Dermatology 82 (4): 1006–1011. doi:10.1016/j.jaad.2019.10.044. PMID 31678467.
- ↑ 27.0 27.1 Scala, E; Cacciapuoti, S; Garzorz-Stark, N; Megna, M; Marasca, C; Seiringer, P; Volz, T; Eyerich, K et al. (15 August 2021). "Hidradenitis Suppurativa: Where We Are and Where We Are Going.". Cells 10 (8): 2094. doi:10.3390/cells10082094. PMID 34440863.
- ↑ "Lithium therapy associated with hidradenitis suppurativa: case report and a review of the dermatologic side effects of lithium". Journal of the American Academy of Dermatology 32 (2 Pt 2): 382–6. February 1995. doi:10.1016/0190-9622(95)90410-7. PMID 7829746.
- ↑ Hidradenitis Suppurativa at eMedicine
- ↑ "The clinical genetics of hidradenitis suppurativa revisited". The British Journal of Dermatology 142 (5): 947–53. May 2000. doi:10.1046/j.1365-2133.2000.03476.x. PMID 10809853.
- ↑ "Androgen metabolism by isolated human axillary apocrine glands in hidradenitis suppurativa". The British Journal of Dermatology 125 (4): 304–8. October 1991. doi:10.1111/j.1365-2133.1991.tb14162.x. PMID 1954117.
- ↑ 32.0 32.1 32.2 Abu Rached, Nessr; Gambichler, Thilo; Ocker, Lennart; Dietrich, Johannes W.; Quast, Daniel R.; Sieger, Christina; Seifert, Caroline; Scheel, Christina et al. (2023-04-01). "Screening for Diabetes Mellitus in Patients with Hidradenitis Suppurativa—A Monocentric Study in Germany" (in en). International Journal of Molecular Sciences 24 (7): 6596. doi:10.3390/ijms24076596. ISSN 1422-0067. PMID 37047569.
- ↑ Reference, Genetics Home. "hidradenitis suppurativa" (in en). https://medlineplus.gov/genetics/condition/hidradenitis-suppurativa/.
- ↑ "A microbiome study to explore the gut-skin axis in hidradenitis suppurativa". Journal of Dermatological Science 101 (3): 218–220. March 2021. doi:10.1016/j.jdermsci.2020.12.008. PMID 33423845. https://pure.eur.nl/en/publications/4cd90f98-29e6-48d3-ad90-b16eeb81184f.
- ↑ "Precocious Puberty More Common in Children with Hidradenitis Suppurativa" (in en). 2024-09-23. https://www.hcplive.com/view/precocious-puberty-more-common-in-children-with-hidradenitis-suppurativa.
- ↑ Mastacouris, Nicole; Midgette, Bria; Strunk, Andrew; Garg, Amit (2024-09-18). "Precocious Puberty Among Children and Adolescents With Hidradenitis Suppurativa". JAMA Dermatology 160 (11): 1245–1246. doi:10.1001/jamadermatol.2024.3104. ISSN 2168-6068. PMID 39292442. PMC 11411440. https://jamanetwork.com/journals/jamadermatology/article-abstract/2823308?&utm_source=BulletinHealthCare&utm_medium=email&utm_term=092424&utm_content=NON-MEMBER&utm_campaign=article_alert-morning_rounds_daily&utm_uid=5590102&utm_effort=DAMR01#google_vignette.
- ↑ 37.0 37.1 37.2 "People with the skin condition, hidradenitis suppurativa, need earlier diagnoses, more treatment options and wider support" (in en). NIHR Evidence (National Institute for Health and Care Research). 2022-02-01. doi:10.3310/alert_48917. https://evidence.nihr.ac.uk/alert/hidradenitis-suppurativa-people-need-earlier-diagnoses-more-treatment-options-wider-support/.
- ↑ 38.0 38.1 38.2 Howells, L.; Lancaster, N.; McPhee, M.; Bundy, C.; Ingram, J.R.; Leighton, P.; Henaghan-Sykes, K.; Thomas, K.S. (29 May 2021). "Thematic synthesis of the experiences of people with hidradenitis suppurativa: a systematic review*" (in en). British Journal of Dermatology 185 (5): 921–934. doi:10.1111/bjd.20523. ISSN 0007-0963. PMID 34050935. https://onlinelibrary.wiley.com/doi/10.1111/bjd.20523.
- ↑ Saunte, D.M.; Boer, J.; Stratigos, A.; Szepietowski, J.C.; Hamzavi, I.; Kim, K.H.; Zarchi, K.; Antoniou, C. et al. (21 July 2015). "Diagnostic delay in hidradenitis suppurativa is a global problem" (in en). British Journal of Dermatology 173 (6): 1546–1549. doi:10.1111/bjd.14038. PMID 26198191. https://onlinelibrary.wiley.com/doi/10.1111/bjd.14038.
- ↑ Giang, Jenny; Seelen, Marc A. J.; van Doorn, Martijn B. A.; Rissmann, Robert; Prens, Errol P.; Damman, Jeffrey (2018). "Complement Activation in Inflammatory Skin Diseases". Frontiers in Immunology 9: 639. doi:10.3389/fimmu.2018.00639. ISSN 1664-3224. PMID 29713318.
- ↑ 41.0 41.1 "HS-USA:: What is Hidradenitis Suppurativa?". http://www.hs-usa.org/hidradenitis_suppurativa.htm.
- ↑ Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH, editors. Dermatologic surgery. Marcel Dekker, New York, 1989, pp. 729–739.
- ↑ "Suggestions for uniform outcome variables when reporting treatment effects in hidradenitis suppurativa". The British Journal of Dermatology 149 (1): 211–3. July 2003. doi:10.1046/j.1365-2133.2003.05390.x. PMID 12890229.
- ↑ "Quality of life impairment in hidradenitis suppurativa: a study of 61 cases". Journal of the American Academy of Dermatology 56 (4): 621–3. April 2007. doi:10.1016/j.jaad.2006.08.061. PMID 17097366.
- ↑ "Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality". The British Journal of Dermatology 174 (5): 970–8. May 2016. doi:10.1111/bjd.14418. PMID 26801356.
- ↑ Sharma, Ruchika (2023-06-01). "Novartis Cosentyx gets European Commission nod for use in adults with hidradenitis suppurativa" (in en). https://medicaldialogues.in/news/industry/pharma/novartis-cosentyx-gets-european-commission-nod-for-use-in-adults-with-hidradenitis-suppurativa-112302.
- ↑ "Non-surgical treatment of hidradenitis suppurativa: the role of cryotherapy". Frontiers in Medicine 10. April 2023. doi:10.3389/fmed.2023.1141691. PMID 37144039.
- ↑ "Hidradenitis suppurativa: A practical review of possible medical treatments based on over 350 hidradenitis patients". Dermatology Online Journal 19 (4): 1. April 2013. doi:10.5070/D35VW402NF. PMID 24021361.
- ↑ "Topical treatment of hidradenitis suppurativa with clindamycin". International Journal of Dermatology 22 (5): 325–8. June 1983. doi:10.1111/j.1365-4362.1983.tb02150.x. PMID 6347922.
- ↑ {{Nosrati A, Ch'en PY, Torpey ME, Shokrian N, Ball G, Benesh G, Andriano TM, Zhu TR, Heibel HD, Hosgood HD, Campton KL, Cohen SR. Efficacy and Durability of Intravenous Ertapenem Therapy for Recalcitrant Hidradenitis Suppurativa. JAMA Dermatol. 2024 Feb 14:e236201. doi: 10.1001/jamadermatol.2023.6201. Epub ahead of print. PMID 38353987; PMCID: PMC10867774.}}
- ↑ 51.0 51.1 Nikolakis, Georgios; Kyrgidis, Athanassios; Zouboulis, Christos C. (2019). "Is There a Role for Antiandrogen Therapy for Hidradenitis Suppurativa? A Systematic Review of Published Data". American Journal of Clinical Dermatology 20 (4): 503–513. doi:10.1007/s40257-019-00442-w. ISSN 1175-0561. PMID 31073704.
- ↑ 52.0 52.1 Goldburg, Samantha R.; Strober, Bruce E.; Payette, Michael J. (2019). "Part 2. Current and emerging treatments for hidradenitis suppurativa". Journal of the American Academy of Dermatology 82 (5): 1061–1082. doi:10.1016/j.jaad.2019.08.089. ISSN 0190-9622. PMID 31604100.
- ↑ Student, Sebastian; Hejmo, Tomasz; Poterała-Hejmo, Aleksandra; Leśniak, Aleksandra; Bułdak, Rafał (2020). "Anti-androgen hormonal therapy for cancer and other diseases". European Journal of Pharmacology 866. doi:10.1016/j.ejphar.2019.172783. ISSN 0014-2999. PMID 31712062.
- ↑ "Etanercept: effective in the management of hidradenitis suppurativa". The British Journal of Dermatology 154 (4): 726–9. April 2006. doi:10.1111/j.1365-2133.2005.07067.x. PMID 16536817.
- ↑ "Treatment of hidradenitis suppurativa with tumour necrosis factor-alpha inhibitors". Acta Dermato-Venereologica 89 (6): 595–600. November 2009. doi:10.2340/00015555-0747. PMID 19997689.
- ↑ "Interventions for Hidradenitis Suppurativa: Updated Summary of an Original Cochrane Review". JAMA Dermatology 153 (5): 458–459. May 2017. doi:10.1001/jamadermatol.2017.0432. PMID 28355440.
- ↑ "FDA approves Novartis Cosentyx® as the first new biologic treatment option for hidradenitis suppurativa patients in nearly a decade". https://www.novartis.com/us-en/news/media-releases/fda-approves-novartis-cosentyx-first-new-biologic-treatment-option-hidradenitis-suppurativa-patients-nearly-decade.
- ↑ "HS Signs and Symptoms | COSENTYX® (Secukinumab)". https://www.cosentyx.com/hidradenitis-suppurativa/signs-and-symptoms.
- ↑ "Updated Physician's Guide to the Off-label Uses of Oral Isotretinoin". The Journal of Clinical and Aesthetic Dermatology 7 (4): 22–34. April 2014. PMID 24765227.
- ↑ "Efficacy of oral zinc and nicotinamide as maintenance therapy for mild/moderate hidradenitis suppurativa: A controlled retrospective clinical study". Journal of the American Academy of Dermatology 83 (2): 665–667. August 2020. doi:10.1016/j.jaad.2020.04.092. PMID 32339699.
- ↑ "Skin-Tissue-sparing Excision with Electrosurgical Peeling (STEEP): a surgical treatment option for severe hidradenitis suppurativa Hurley stage II/III". Journal of the European Academy of Dermatology and Venereology 29 (2): 379–382. February 2015. doi:10.1111/jdv.12376. PMID 24460855.
- ↑ "Hidradenitis suppurativa: A practical review of possible medical treatments based on over 350 hidradenitis patients". Dermatology Online Journal 19 (4): 1. April 2013. doi:10.5070/D35VW402NF. PMID 24021361. http://escholarship.org/uc/item/5vw402nf. Retrieved 4 March 2014.
- ↑ "Histopathologic study of hidradenitis suppurativa following long-pulsed 1064-nm Nd:YAG laser treatment". Archives of Dermatology 147 (1): 21–8. January 2011. doi:10.1001/archdermatol.2010.245. PMID 20855672.
- ↑ "Botulinum Toxin in Hidradenitis Suppurativa: A Systematic Review". Journal of Drugs in Dermatology 21 (4): 408–412. 2022. PMID 35389587.
- ↑ "[Squamous cell carcinoma arising in Verneuil's disease: two cases and literature review]" (in fr). Annales de Chirurgie Plastique et Esthétique 51 (1): 82–6. February 2006. doi:10.1016/j.anplas.2005.11.002. PMID 16488526.
- ↑ "Vulval squamous cell carcinoma arising in chronic hidradenitis suppurativa". Clinical and Experimental Dermatology 30 (5): 481–3. September 2005. doi:10.1111/j.1365-2230.2005.01875.x. PMID 16045671.
- ↑ Abu Rached, N; Gambichler, T; Dietrich, JW; Ocker, L; Seifert, C; Stockfleth, E; Bechara, FG (3 December 2022). "The Role of Hormones in Hidradenitis Suppurativa: A Systematic Review.". International Journal of Molecular Sciences 23 (23): 15250. doi:10.3390/ijms232315250. PMID 36499573.
- ↑ 68.0 68.1 Abu Rached, N; Dietrich, JW; Ocker, L; Quast, DR; Stockfleth, E; Bechara, FG (6 August 2024). "Diabetes remission associated with optimized treatment of hidradenitis suppurativa.". Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology 22 (10): 1427–1429. doi:10.1111/ddg.15490. PMID 39106221.
- ↑ Abu Rached, N; Dietrich, JW; Ocker, L; Quast, DR; Scheel, C; Gambichler, T; Bechara, FG (4 December 2023). "Primary Thyroid Dysfunction Is Prevalent in Hidradenitis Suppurativa and Marked by a Signature of Hypothyroid Graves' Disease: A Case-Control Study.". Journal of Clinical Medicine 12 (23): 7490. doi:10.3390/jcm12237490. PMID 38068542.
- ↑ Abu Rached, Nessr; Dietrich, Johannes W.; Ocker, Lennart; Stockfleth, Eggert; Haven, Yannik; Myszkowski, Daniel; Bechara, Falk G. (21 March 2025). "Endotyping Insulin–Glucose Homeostasis in Hidradenitis Suppurativa: The Impact of Diabetes Mellitus and Inflammation". Journal of Clinical Medicine 14 (7): 2145. doi:10.3390/jcm14072145. PMID 40217596.
- ↑ Phan, K; Charlton, O; Smith, SD (February 2020). "Hidradenitis suppurativa and polycystic ovarian syndrome: Systematic review and meta-analysis". The Australasian Journal of Dermatology 61 (1): e28-33. doi:10.1111/ajd.13110. PMID 31261440.
- ↑ 72.0 72.1 72.2 72.3 72.4 72.5 Garg, A; Malviya, N; Strunk, A; Wright, S; Alavi, A; Alhusayen, R; Alikhan, A; Daveluy, SD et al. (May 2022). "Comorbidity screening in hidradenitis suppurativa: Evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations". Journal of the American Academy of Dermatology 86 (5): 1092–1101. doi:10.1016/j.jaad.2021.01.059. PMID 33493574. PMC 8298595. https://www.jaad.org/article/S0190-9622(21)00213-9/fulltext. Retrieved 10 April 2025.
- ↑ Hurley HJJ. Apocrine glands. New York: McGraw Hill; 1979.
- ↑ "Anemia associated with hidradenitis suppurativa". Archives of Dermatology 98 (2): 138–40. August 1968. doi:10.1001/archderm.98.2.138. PMID 5667225.
- ↑ "Risk of inflammatory arthritis after a new diagnosis of hidradenitis suppurativa". JAMA Dermatology 156 (3): 342–345. 2020. doi:10.1001/jamadermatol.2019.4590. PMID 31968066.
- ↑ "Perineal hidradenitis suppurativa: presentation of two unusual complications and a review". Annals of Plastic Surgery 26 (5): 456–62. May 1991. doi:10.1097/00000637-199105000-00008. PMID 1952719.
- ↑ "Incidence of cancer among patients with hidradenitis suppurativa". Archives of Dermatology 137 (6): 730–4. June 2001. PMID 11405761.
- ↑ "Hidradenitis suppurativa. Complications resulting in death". JAMA 198 (1): 201–3. October 1966. doi:10.1001/jama.198.1.201. PMID 5953172.
- ↑ "Immunological studies of the arthritis of acne conglobata and hidradenitis suppurativa". Clinical and Experimental Rheumatology 2 (4): 309–11. 1984. PMID 6241861.
- ↑ Jalenques, Isabelle; Ciortianu, Laura; Pereira, Bruno; D'Incan, Michel; Lauron, Sophie; Rondepierre, Fabien (25 March 2020). "The prevalence and odds of anxiety and depression in children and adults with hidradenitis suppurativa: Systematic review and meta-analysis" (in en). Journal of the American Academy of Dermatology 83 (2): 542–553. doi:10.1016/j.jaad.2020.03.041. PMID 32222447.
- ↑ 81.0 81.1 81.2 81.3 Ingram, John R. (3 September 2020). "The epidemiology of hidradenitis suppurativa*" (in en). British Journal of Dermatology 183 (6): 990–998. doi:10.1111/bjd.19435. ISSN 0007-0963. PMID 32880911.
- ↑ Velpeau A. Aissele. In: Bechet Jeune Z: Dictionnaire de médecine, on Repertoire Générale des Sciences Medicals sous le Rapport Theorique et Pratique. 1839.
- ↑ Verneuil AS (1854). "Etudes sur les tumeurs de la peau et quelques maladies de glandes sudoripares" (in fr). Archives of General Medicine 94: 693–705.
- ↑ Die Hautdrüsen der Menschen und der Säugetiere, ihre histologische und rassenanatomische Bedeutung sowie die muscularis sexualis. Stuttgart: Schweizerbart E. 1922.
- ↑ Pillsbury DM, ed (1956). "Bacterial infections of the skin". Dermatoloy (1st ed.). Philadelphia. pp. 482–9.
- ↑ Triads in Dermatology; Prachi G Agrawal, Uday S Khopkar, [...], and Sunil N Mishra; Indian J Dermatol. 2013 Sep–Oct; 58(5): 346–351
- ↑ Plewig, Gerd; Kligman, Albert M. (1975). "Acne Conglobata". in Plewig, Gerd; Kligman, Albert M.. Acne. pp. 168–203. doi:10.1007/978-3-642-96246-2_11. ISBN 978-3-642-96246-2.
- ↑ 88.0 88.1 "Acne inversa (alias acne triad, acne tetrad or hidradenitis suppurativa)". Acne and Related Disorders. London: Martin Dunitz. 1989. pp. 345–57.
- ↑ ""Hidradenitis suppurativa" is acne inversa! An appeal to (finally) abandon a misnomer". International Journal of Dermatology 44 (7): 535–40. July 2005. doi:10.1111/j.1365-4632.2004.02536.x. PMID 15985019.
- ↑ "Hidradenitis suppurativa is acne inversa". International Journal of Dermatology 46 (3): 330; author reply 330–2. March 2007. doi:10.1111/j.1365-4632.2007.02872.x. PMID 17343599.
- ↑ "Hidradenitis should not be renamed acne inversa". Dermatology Online Journal 12 (7): 6. December 2006. doi:10.5070/D35G21T4MQ. PMID 17459292.
- ↑ Verneuil AS (1854). "Etudes sur les tumor de la peau" (in fr). Arch Gen Med 94: 693.
- ↑ "Hidradenitis suppurativa: a disease of follicular epithelium, rather than apocrine glands". The British Journal of Dermatology 122 (6): 763–9. June 1990. doi:10.1111/j.1365-2133.1990.tb06264.x. PMID 2369556.
- ↑ "Hidradenitis suppurativa or acne inversa. A clinicopathological study of early lesions". The British Journal of Dermatology 135 (5): 721–5. November 1996. doi:10.1111/j.1365-2133.1996.tb03880.x. PMID 8977671.
- ↑ van Straalen, KR; Prens, EP; Gudjonsson, JE (April 2022). "Insights into hidradenitis suppurativa.". The Journal of Allergy and Clinical Immunology 149 (4): 1150–1161. doi:10.1016/j.jaci.2022.02.003. PMID 35189127.
External links
- Hidradenitis suppurativa at American Academy of Dermatology Association
- Hidradenitis suppurativa at British Association of Dermatologists
- Hidradenitis Suppurativa Foundation
| Classification | |
|---|---|
| External resources |
 |