Chemistry:Infliximab
 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Chimeric (mouse/human) |
| Target | Tumor necrosis factors (TNF) |
| Clinical data | |
| Trade names | Remicade, others |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous, subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 92% (IV, if 8% left in the syringe) |
| Metabolism | reticuloendothelial system |
| Elimination half-life | 9.5 days |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C6428H9912N1694O1987S46 |
| Molar mass | 144190.64 g·mol−1 |
| | |
Infliximab, a chimeric monoclonal antibody, sold under the brand name Remicade among others, is a medication used to treat a number of autoimmune diseases. This includes Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis, and Behçet's disease.[3] It is given by slow injection into a vein, typically at six- to eight-week intervals.[3]
Common side effects include infections, acute infusion reactions, and abdominal pain.[3] Infliximab is a chimeric monoclonal antibody biologic. It seems to work by binding to and neutralizing TNF-α, preventing it from interacting with its receptors on the cell.[3] TNF-α is a chemical messenger (cytokine) and a key part of the autoimmune reaction.
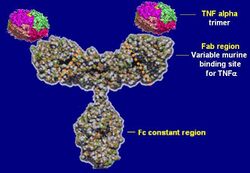
Infliximab was originally developed in mice as a mouse antibody. Because humans have immune reactions to mouse proteins, the mouse common domains were replaced with similar human antibody domains. They are monoclonal antibodies and have identical structures and affinities to the target. Because they are a combination of mouse and human antibody amino acid sequences, they are called a "chimeric monoclonal antibody".[4]
Infliximab was approved for medical use in the United States in 1998,[3] and in the European Union in August 1999.[5] Infliximab biosimilars have been approved in the EU (2013), in Japan (2014), and in the United States (2016, 2017, 2019).[6][7][8] It is on the World Health Organization's List of Essential Medicines.[9]
Medical uses
Crohn's disease
Three phenotypes, or categories of disease, are present in Crohn's disease: stricturing disease (which causes narrowing of the bowel), penetrating disease (which causes fistulae or abnormal connections of the bowel), and inflammatory disease (which primarily causes inflammation).[10]
Fistulizing disease
Infliximab was first used for closure of fistulae in Crohn's disease in 1999. In a 94-patient, phase II clinical trial, the researchers showed Infliximab was effective in closing fistulae between the skin and bowel in 56–68% of patients.[11] A large, 296-patient Phase III clinical trial called the ACCENT 2 trial showed infliximab was additionally beneficial in maintaining closure of fistulae, with almost two-thirds of all patients treated with the three initial doses of infliximab having a fistula response after 14 weeks, and 36% of patients maintaining closure of fistulae after a year, compared with 19% who received placebo therapy. This final trial resulted in the FDA approval of the drug to treat fistulizing disease.[12]
Inflammatory disease
Infliximab has been used to induce and maintain remission in inflammatory Crohn's disease. The ACCENT 1 trial, a large, multicentre trial, found 39–45% of patients treated with infliximab, who had an initial response to it, maintained remission after 30 weeks, compared with 21% who received placebo treatment. It also showed a mean maintenance of remission from 38 to 54 weeks compared with 21 weeks for patients who received placebo treatment.[13]
Crohn's patients have flares of their disease between periods of disease quiescence. Severe flares are usually treated with steroid medications to obtain remission, but steroids have many undesirable side effects, so some gastroenterologists are now advocating the use of infliximab as the first drug to try to get patients into remission. This has been called the top-down approach to treatment.[14]
Ulcerative colitis
Infliximab targets TNF, thought to be more related to Th1 cytokines. Ulcerative colitis was thought to be a Th2 disease, and infliximab would be of limited use. However, patients with ulcerative colitis have begun to be treated with infliximab on the basis of two large clinical trials conducted in 2005 by Paul Rutgeerts and William Sandborn. The Acute ulcerative Colitis Treatment trials (ACT1 and ACT2) to evaluate the utility of infliximab in ulcerative colitis showed 44–45% of patients treated with infliximab for a year maintained a response to the medication, compared with 21% of patients who were treated with placebo medication. At two months, the response was 61–69% for patients treated with infliximab, and 31% for those treated with placebo.[15]
Psoriatic arthritis
In psoriatic arthritis (PsA), inhibitors of TNF, such as infliximab, improve the signs and symptoms. Several therapies with modest efficacy have been studied in nail psoriasis. Among available agents, higher quality data are available to support the efficacy of cyclosporine and infliximab. Based on studies in AS, the results suggest infliximab, etanercept, and adalimumab have the potential to reduce the signs and symptoms of moderate to severely active axial involvement in PsA in patients who have had an inadequate response to NSAID (level 1a, grade A). The anti-TNF agents (infliximab and etanercept; level 1b, grade A) are more effective for the treatment of enthesitis than traditional agents. Results suggest infliximab is effective for the treatment of dactylitis in PsA.[16]
Other
It was approved for treating ankylosing spondylitis,[17] psoriatic arthritis, psoriasis, rheumatoid arthritis.[18]
Infliximab is also prescribed (out of indication) for the treatment of Behçet's disease.[19]
Infliximab is the most frequently used biological agent in treating relapsing polychondritis.[20] Half of the patients saw benefit from this treatment, and a few other patients experienced infections that in some cases lead to death.[20][21]
There have been numerous case reports of the efficacy of infliximab in various inflammatory skin conditions diseases; the FDA approved infliximab for chronic severe plaque psoriasis in adults in September 2006.[22]
Infliximab has been used off-label in treating refractory sarcoidosis, where other treatments have not been effective.[23]
Infliximab has been tested in chronic obstructive pulmonary disease (COPD) but there was no evidence of benefit with the possibility of harm.[24]
Infliximab is indicated for steroid refractory checkpoint inhibitor induced colitis, at a dose of 5 to 10 mg/kg.[25]
Adverse effects
Infliximab has adverse effects, some life-threatening, common to drugs in the class of TNF inhibiting immunosuppressants (which also includes etanercept (Enbrel) and adalimumab (Humira)). Some of the most severe are:
- serious infections
- reactivation of hepatitis B
- reactivation of tuberculosis[26]
- lethal hepatosplenic T-cell lymphoma (generally only when combined with 6-mercaptopurine)
- drug-induced lupus
- demyelinating central nervous system disorders
- psoriasis and psoriasiform skin lesions
- new-onset vitiligo
Cases of leukopenia, neutropenia, thrombocytopenia, and pancytopenia (some fatal) have been reported with infliximab.[27] The FDA issued a warning to doctors appearing in the respective product labeling of infliximab instructing them to screen and monitor potential patients more carefully.[28] The FDA issued a warning to doctors that there is an increased risk of lymphoma and other cancers associated with the use of infliximab and other tumor necrosis factor blockers in children and adolescents.[29]
Maintenance therapy with the drug (versus intermittent or sporadic therapy) lessens the likelihood of developing antibodies to infliximab which are known to reduce the efficacy of the drug. Combination treatment with methotrexate (an antifolate drug which suppresses the immune system) has been shown to reduce the formation of these antibodies in patients with rheumatoid arthritis[30] and combination therapy with other immunosuppressants has been shown to reduce the likelihood of these antibodies being formed in Crohn's disease.[13] The use of immunosuppressants may not be necessary in all diseases for which infliximab is indicated, and indiscriminant uses of these other immunosuppressants carry their own risks. Infliximab was studied in monotherapy (without concomitant immunosuppressants such as methotrexate or azathioprine) in psoriasis, psoriatic arthritis, and ankylosing spondylitis.[17] Only its use in rheumatoid arthritis requires the concomitant use of methotrexate by the U.S. Food and Drug Administration (FDA) product labeling; however, the concomitant use of methotrexate in other disease states may help to reduce the body's immune response to the infliximab and increase its duration of efficacy.
Pharmacology
Infliximab is a purified, recombinant DNA-derived chimeric human-mouse IgG monoclonal antibody that consists of mouse heavy and light chain variable regions combined with human heavy and light chain constant regions.[31] It has a serum half-life of 9.5 days and can be detected in serum 8 weeks after infusion treatment.[31]
Infliximab neutralizes the biological activity of TNF-α by binding with high affinity to the soluble (free floating in the blood) and transmembrane (located on the outer membranes of T cells and similar immune cells) forms of TNF-α, and inhibits or prevents the effective binding of TNF-α with its receptors. Infliximab and adalimumab (another TNF antagonist) are in the subclass of "anti-TNF antibodies" (they are in the form of naturally occurring antibodies), and are capable of neutralizing all forms (extracellular-, transmembrane-, and receptor-bound) of TNF-α.[32] Etanercept, a third TNF antagonist, is in a different subclass (receptor-construct fusion protein), and, because of its modified form, cannot neutralize receptor-bound TNF-α.[33] Additionally, the anti-TNF antibodies adalimumab and infliximab have the capability of lysing cells involved in the inflammatory process, whereas the receptor fusion protein apparently lacks this capability.[34] Although the clinical significance of these differences have not been absolutely proven, etanercept, has been shown to perform worse than placebo for Crohn's disease. These differences may account for the differential actions of these drugs in both efficacy and side effects.
Infliximab has high specificity for TNF-α, and does not neutralize TNF beta (TNFβ, also called lymphotoxin α), an unrelated cytokine that uses different receptors from TNF-α. Biological activities attributed to TNF-α (and therefore potentially inhibited by Infliximab) include induction of proinflammatory cytokines (such as interleukins IL-1 and IL-6), enhancement of leukocyte movement or migration from the blood vessels into the tissues (by increasing the permeability of endothelial layer of blood vessels), and increasing the release of adhesion molecules. Infliximab prevents TNF-α induced disease in transgenic mice (a special type of mice biologically engineered to produce a human form of TNF-α) that are used to test how these drugs that might be expected to affect humans. These experimental mice develop arthritis as a result of their production of human TNF-α, and when administered after disease onset, infliximab allows damaged joints to heal.
Other monoclonal antibodies targeting TNF-α are golimumab, adalimumab, and certolizumab pegol. Etanercept also binds and inhibits the action of TNF-α, but is not a monoclonal antibody (it is instead a fusion of TNF-receptor and an antibody constant region).[35]
History
The importance of TNF in the development of rheumatoid arthritis was originally demonstrated by George Kollias and colleagues in proof of principle studies in transgenic animal models.[36][37]
Infliximab was developed by Junming Le (b. 1940) and Jan Vilček (b. 1933) at New York University School of Medicine and in collaboration with Centocor (now Janssen Biotech, Inc.).[38]
Society and culture
Marketing
Remicade is marketed by Janssen Biotech, Inc. (formerly Centocor Biotech, Inc.) in the United States, Mitsubishi Tanabe Pharma in Japan, Xian Janssen in China, and Schering-Plough (now part of Merck & Co) elsewhere.[39]
Biosimilars
In June 2013, two biosimilar versions (Inflectra and Remsima) were submitted for approval in Europe, by Hospira and Celltrion Healthcare respectively.[40] Both had a positive opinion from European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP) for sale in the European Union (EU).[41] Celltrion obtained marketing authorization approval (MAA) from 27 EU countries and 3 EEA (European Economic Area) countries by September 2013.[42][43][44] Inflectra was approved for use in the European Union in September 2013,[44] and Remsima was approved for use in the European Union in October 2013.[43]
In Japan, Celltrion received marketing authorization for Remsima from Japan's Ministry of Health, Labour and Welfare (MHLW) in July 2014.[citation needed]
In India, Epirus Biopharmaceuticals obtained approval to produce biosimilar infliximab under the brand name "Infimab" (trail name BOW015).[45]
The U.S. Food and Drug Administration (FDA) approved Celltrion/Hospira/Pfizer's Inflectra (infliximab-dyyb) in April 2016.[46][47] Although the US brand name is Inflectra, it is marketed in the European Union as Remsima.[43]
The FDA approved Samsung Bioepis Co., Ltd.'s Renflexis (infliximab-abda) in April 2017.[6]
Biogen released another biosimilar, Flixabi, which was approved in Germany, the UK, and the Netherlands.[48] Flixabi was approved for use in the European Union in May 2016.[49]
In December 2017, Ixifi (infliximab-qbtx) was approved in the United States.[7]
Zessly was approved for use in the European Union in May 2018.[50]
In December 2019, Avsola (infliximab-axxq) was approved in the United States.[8]
Avsola was approved for medical use in Canada in March 2020.[51]
In December 2021, Ixifi was approved for medical use in Canada.[52]
Availability/affordability
In the United States, infliximab is an expensive medication, costing about US$900 for a 100 mg dose, and is covered by almost every US medical insurance plan (though caps on many plans make it possible to be covered for only a subset of treatments in the course of a year).[citation needed] Infliximab is supplied as a sterile, white, lyophilized (freeze-dried) powder,[53] so must be reconstituted and administered by a health care professional, usually in a hospital or office setting.[39] For this reason, it is usually covered under major medical insurance rather than prescription drug coverage. The loading regimen for all approved indications occurs at weeks 0, 2, and 6 at the above dosages.[39]
In the UK, infliximab is available from the NHS for Crohn's disease treatment provided three criteria are met.[54] Patients should have severe active Crohn's disease with a CDAI score of 300 or more, have not responded to immunomodulating drugs and corticosteroids, and for whom surgery is inappropriate. Since February 2015, it is also approved for the treatment of ulcerative colitis where other treatments have not worked.[55]
In Australia, infliximab is available through the PBS for Crohn's disease treatment provided the patient has not responded to conventional treatment and has a severe case of the condition.[56]
Infliximab is available in the Republic of Ireland through the HSE's Medical Card and Drug Payment Scheme.[citation needed]
Johnson & Johnson reported in its 2013 annual report, "Remicade (infliximab), accounted for approximately 9.4% of the Company's total revenues for fiscal 2013."[57]
Zymfentra was approved for medical use in the United States in October 2023.[2]
References
- ↑ "Infliximab Use During Pregnancy". 2 July 2019. https://www.drugs.com/pregnancy/infliximab.html.
- ↑ 2.0 2.1 "Celltrion USA Announces U.S. FDA Approval of Zymfentra (infliximab-dyyb), the First and Only Subcutaneous infliximab, for the Treatment of People With Inflammatory Bowel Disease". Celltrion. 22 October 2023. https://www.businesswire.com/news/home/20231022057773/en/Celltrion-USA-Announces-U.S.-FDA-Approval-of-ZYMFENTRA%C2%AE-infliximab-dyyb-the-First-and-Only-Subcutaneous-infliximab-for-the-Treatment-of-People-With-Inflammatory-Bowel-Disease/.
- ↑ 3.0 3.1 3.2 3.3 3.4 "Infliximab, Infliximab-dyyb Monograph for Professionals". American Society of Health-System Pharmacists. https://www.drugs.com/monograph/infliximab-infliximab-dyyb.html.
- ↑ "Infliximab: a novel chimeric monoclonal antibody for the treatment of Crohn's disease". Clinical Therapeutics 21 (6): 932–42; discussion 931. June 1999. doi:10.1016/s0149-2918(99)80015-0. PMID 10440618.
- ↑ "Remicade EPAR". https://www.ema.europa.eu/en/medicines/human/EPAR/remicade.
- ↑ 6.0 6.1 "Renflexis: FDA Approved Drug Products". 17 April 2019. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761054.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 7.0 7.1 "Ixifi: FDA-Approved Drugs". 6 December 2019. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761072.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 8.0 8.1 "Avsola: FDA-Approved Drugs". 6 December 2019. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761086.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ "Treatment of Crohn's Disease of Inflammatory, Stenotic, and Fistulizing Phenotypes". Curr Treat Options Gastroenterol 6 (3): 183–200. June 2003. doi:10.1007/s11938-003-0001-1. PMID 12744819.
- ↑ "Infliximab for the treatment of fistulas in patients with Crohn's disease". The New England Journal of Medicine 340 (18): 1398–405. May 1999. doi:10.1056/NEJM199905063401804. PMID 10228190. https://pure.uva.nl/ws/files/3156092/7456_77284y.pdf.
- ↑ "Infliximab maintenance therapy for fistulizing Crohn's disease". The New England Journal of Medicine 350 (9): 876–85. February 2004. doi:10.1056/NEJMoa030815. PMID 14985485.
- ↑ 13.0 13.1 "Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial". Lancet 359 (9317): 1541–9. May 2002. doi:10.1016/S0140-6736(02)08512-4. PMID 12047962.
- ↑ "Crohn's disease: step up or top down therapy". Best Pract Res Clin Gastroenterol 17 (1): 131–7. February 2003. doi:10.1053/bega.2003.0361. PMID 12617888.
- ↑ "Infliximab for induction and maintenance therapy for ulcerative colitis". The New England Journal of Medicine 353 (23): 2462–76. 2005. doi:10.1056/NEJMoa050516. PMID 16339095.
- ↑ "Systematic review of treatments for psoriatic arthritis: an evidence based approach and basis for treatment guidelines". J. Rheumatol. 33 (7): 1417–21. July 2006. PMID 16724373.
- ↑ 17.0 17.1 "TNF-alpha inhibitors for ankylosing spondylitis". The Cochrane Database of Systematic Reviews 4 (4): CD005468. April 2015. doi:10.1002/14651858.CD005468.pub2. PMID 25887212.
- ↑ "Infliximab for the treatment of rheumatoid arthritis". The Cochrane Database of Systematic Reviews 2002 (3): CD003785. 2002-07-22. doi:10.1002/14651858.cd003785. PMID 12137712.
- ↑ "Behçet's disease: a new target for anti-tumour necrosis factor treatment". Ann. Rheum. Dis. 61 (Suppl 2): ii51–3. November 2002. doi:10.1136/ard.61.suppl_2.ii51. PMID 12379622.
- ↑ 20.0 20.1 "Relapsing polychondritis". Joint Bone Spine 81 (2): 118–24. March 2014. doi:10.1016/j.jbspin.2014.01.001. PMID 24556284. http://www.hal.inserm.fr/inserm-00951203.
- ↑ "Biologics in relapsing polychondritis: a literature review". Seminars in Arthritis and Rheumatism 41 (5): 712–9. April 2012. doi:10.1016/j.semarthrit.2011.08.006. PMID 22071463.
- ↑ "A review of the use of infliximab to manage cutaneous dermatoses". J Cutan Med Surg 8 (2): 77–89. 2004. doi:10.1007/s10227-004-0115-7. PMID 15685387.
- ↑ "Treatment of Sarcoidosis". Clinics in Chest Medicine 36 (4): 751–767. December 2015. doi:10.1016/j.ccm.2015.08.015. PMID 26593147.
- ↑ "The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease". Am. J. Respir. Crit. Care Med. 175 (9): 926–34. May 2007. doi:10.1164/rccm.200607-995OC. PMID 17290043.
- ↑ "Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline". Journal of Clinical Oncology 36 (17): 1714–1768. June 2018. doi:10.1200/JCO.2017.77.6385. PMID 29442540.
- ↑ "Tuberculosis associated with infliximab, a tumor necrosis factor α–neutralizing agent". N Engl J Med 345 (15): 1098–1104. 2001. doi:10.1056/NEJMoa011110. PMID 11596589.
- ↑ "Remicade for Healthcare Professionals". http://www.remicade.com/remicade/global/hcp/healthcare_professionals.html.
- ↑ "REMICADE® (infliximab) for IV Injection". Centocor, Inc.. U.S. Food and Drug Administration. May 2006. https://www.fda.gov/medwatch/safety/2006/May_PIs/Remicade_PI.pdf.
- ↑ "Tumor Necrosis Factor (TNF) Blockers (marketed as Remicade, Enbrel, Humira, Cimzia, and Simponi) August 2009". MedWatch. U.S. Food and Drug Administration (FDA). August 31, 2009. https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalproducts/ucm175843.htm.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group". Lancet 354 (9194): 1932–9. Dec 4, 1999. doi:10.1016/s0140-6736(99)05246-0. PMID 10622295.
- ↑ 31.0 31.1 "Promising biological therapies for ulcerative colitis: A review of the literature". World Journal of Gastrointestinal Pathophysiology 6 (4): 219–227. November 2015. doi:10.4291/wjgp.v6.i4.219. PMID 26600980.
- ↑ "Cytokine pathways and joint inflammation in rheumatoid arthritis". The New England Journal of Medicine 344 (12): 907–16. March 2001. doi:10.1056/NEJM200103223441207. PMID 11259725.
- ↑ Etanercept product labeling
- ↑ Etanercept, Adalimumab and Infliximab product labeling
- ↑ "A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity". J. Exp. Med. 174 (6): 1483–9. 1991. doi:10.1084/jem.174.6.1483. PMID 1660525.
- ↑ "Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis". The EMBO Journal 10 (13): 4025–31. December 1991. doi:10.1002/j.1460-2075.1991.tb04978.x. PMID 1721867.
- ↑ "Regulation of tumour necrosis factor signalling: live or let die.". Nat Rev Immunol 15 (6): 362–74. May 2015. doi:10.1038/nri3834. PMID 26008591.
- ↑ "Construction and initial characterization of a mouse-human chimeric anti-TNF antibody". Mol. Immunol. 30 (16): 1443–53. November 1993. doi:10.1016/0161-5890(93)90106-L. PMID 8232330.
- ↑ 39.0 39.1 39.2 "Remicade Becomes First Anti-TNF Biologic Therapy to Treat One Million Patients Worldwide" (Press release). Johnson & Johnson. November 6, 2007. Archived from the original on July 13, 2011. Retrieved 2009-11-14.
- ↑ "Billion-dollar biotech drug may soon have biosimilar competition". Philadelphia Business Journal. June 28, 2013. http://www.bizjournals.com/philadelphia/news/2013/06/28/biliion-dollar-biotech-drug-may-soon.html.
- ↑ "European Medicines Agency recommends approval of first two monoclonal-antibody biosimilars". European Medicines Agency. June 28, 2013. http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2013/06/news_detail_001837.jsp&mid=WC0b01ac058004d5c1.
- ↑ "European Commission Approves Biosimilar of J&J and Merck's Remicade". 10 September 2013. https://www.wsj.com/articles/european-commission-approves-biosimilar-of-jj-and-mercks-remicade-1378844400.
- ↑ 43.0 43.1 43.2 "Remsima EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/remsima.
- ↑ 44.0 44.1 "Inflectra EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/inflectra.
- ↑ "Epirus racks up its first biosimilar approval in India". http://www.bioworld.com/content/epirus-racks-its-first-biosimilar-approval-india-0.
- ↑ "FDA approves Inflectra, a biosimilar to Remicade". U.S. Food and Drug Administration (FDA) (Press release). 6 December 2019. Archived from the original on 7 December 2019. Retrieved 6 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Inflectra: FDA-Approved Drugs". 6 December 2019. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=125544.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Key Takeaways from Biogen's Q3 Call: Tecfidera, Pipeline, Biosimilars". 27 October 2016. http://www.nasdaq.com/article/key-takeaways-from-biogens-q3-call-tecfidera-pipeline-biosimilars-cm699225/.
- ↑ "Flixabi EPAR". https://www.ema.europa.eu/en/medicines/human/EPAR/flixabi.
- ↑ "Zessly EPAR". https://www.ema.europa.eu/en/medicines/human/EPAR/zessly.
- ↑ "Summary Basis of Decision (SBD) for Avsola". 23 October 2014. https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00478&lang=en.
- ↑ "Summary Basis of Decision (SBD) for Ixifi". 23 October 2014. https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?linkID=SBD00583&lang=en.
- ↑ "Remicade (Infliximab): Side Effects, Interactions, Warning, Dosage & Uses". http://www.rxlist.com/remicade-drug.htm.
- ↑ "The clinical effectiveness and cost effectiveness of infliximab for Crohn's disease - Guidance and guidelines - NICE". http://guidance.nice.org.uk/TA40/Guidance.
- ↑ "Infliximab, adalimumab and golimumab for treating moderately to severely active ulcerative colitis after the failure of conventional therapy". http://www.nice.org.uk/guidance/ta329.
- ↑ "Section 100 arrangements; only for infliximab". http://www.medicareaustralia.gov.au/provider/pbs/drugs1/crohns.jsp#N10041.
- ↑ JNJ annual report, downloaded April 22, 2014. "2013 1229 10-K". https://www.sec.gov/Archives/edgar/data/200406/000020040614000033/a2013122910-k.htm.
 |
