Medicine:Sarcoidosis
| Sarcoidosis | |
|---|---|
| Other names | Sarcoïdosis, sarcoid, Besnier–Boeck–Schaumann disease[1] |
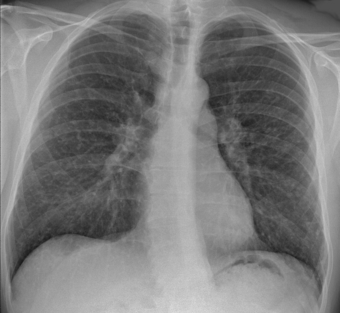 | |
| Chest X-ray showing the typical nodularity of sarcoidosis, predominantly in the hila of the lungs. | |
| Pronunciation |
|
| Specialty | Rheumatology, Immunology |
| Symptoms | |
| Usual onset | 20–50 years old More common in women[4] |
| Duration | Few years to long term[2][5] |
| Causes | Unknown[2] |
| Risk factors | Family history[4] |
| Diagnostic method | Based on symptoms and tissue biopsy[6] |
| Differential diagnosis | Tuberculosis, lymphoma, infectious mononucleosis, pulmonary eosinophilia[7] |
| Treatment | Ibuprofen, prednisone, methotrexate[8][9] |
| Prognosis | Mortality 1–7%[5] |
| Frequency | 1.9 million with interstitial lung disease (2015)[10] |
| Deaths | 122,000 with interstitial lung disease (2015)[11] |
Sarcoidosis (also known as Besnier–Boeck–Schaumann disease) is a disease involving abnormal collections of inflammatory cells that form lumps known as granulomata.[2] The disease usually begins in the lungs, skin, or lymph nodes.[2] Less commonly affected are the eyes, liver, heart, and brain, though any organ can be affected.[2] The signs and symptoms depend on the organ involved.[2] Often, no, or only mild, symptoms are seen.[2] When it affects the lungs, wheezing, coughing, shortness of breath, or chest pain may occur.[3] Some may have Löfgren syndrome with fever, large lymph nodes, arthritis, and a rash known as erythema nodosum.[2]
The cause of sarcoidosis is unknown.[2] Some believe it may be due to an immune reaction to a trigger such as an infection or chemicals in those who are genetically predisposed.[12][13] Those with affected family members are at greater risk.[4] Diagnosis is partly based on signs and symptoms, which may be supported by biopsy.[6] Findings that make it likely include large lymph nodes at the root of the lung on both sides, high blood calcium with a normal parathyroid hormone level, or elevated levels of angiotensin-converting enzyme in the blood.[6] The diagnosis should be made only after excluding other possible causes of similar symptoms such as tuberculosis.[6]
Sarcoidosis may resolve without any treatment within a few years.[2][5] However, some people may have long-term or severe disease.[5] Some symptoms may be improved with the use of anti-inflammatory drugs such as ibuprofen.[8] In cases where the condition causes significant health problems, steroids such as prednisone are indicated.[9] Medications such as methotrexate, chloroquine, or azathioprine may occasionally be used in an effort to decrease the side effects of steroids.[9] The risk of death is 1–7%.[5] The chance of the disease returning in someone who has had it previously is less than 5%.[2]
In 2015, pulmonary sarcoidosis and interstitial lung disease affected 1.9 million people globally and they resulted in 122,000 deaths.[10][11] It is most common in Scandinavians, but occurs in all parts of the world.[14] In the United States, risk is greater among black people as opposed to white people.[14] It usually begins between the ages of 20 and 50.[4] It occurs more often in women than men.[4] Sarcoidosis was first described in 1877 by the English doctor Jonathan Hutchinson as a non-painful skin disease.[15]
Signs and symptoms

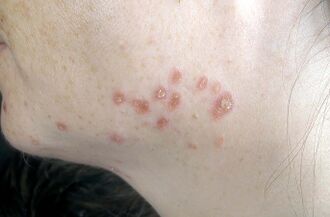
Sarcoidosis is a systemic inflammatory disease that can affect any organ, although it can be asymptomatic and is discovered by accident in about 5% of cases.[17] Common symptoms, which tend to be vague, include fatigue (unrelieved by sleep; occurs in up to 85% of cases [18]), lack of energy, weight loss, joint aches and pains (which occur in about 70% of cases),[19] arthritis (14–38% of cases), dry eyes, swelling of the knees, blurry vision, shortness of breath, a dry, hacking cough, or skin lesions.[20][21][22][23] Less commonly, people may cough up blood.[20] Sarcoidosis is also accompanied by psychological distress and symptoms of anxiety and depression, which are also associated with fatigue.[24] The cutaneous symptoms vary, and range from rashes and noduli (small bumps) to erythema nodosum, granuloma annulare, or lupus pernio. Sarcoidosis and cancer may mimic one another, making the distinction difficult.[25]
The combination of erythema nodosum, bilateral hilar lymphadenopathy, and joint pain is called Löfgren syndrome, which has a relatively good prognosis.[20] This form of the disease occurs significantly more often in Scandinavian patients than in those of non-Scandinavian origin.[26]
Respiratory tract
Localization to the lungs is by far the most common manifestation of sarcoidosis.[27] At least 90% of those affected experience lung involvement.[28] Overall, about 50% develop permanent pulmonary abnormalities, and 5 to 15% have progressive fibrosis of the lung parenchyma. Sarcoidosis of the lung is primarily an interstitial lung disease in which the inflammatory process involves the alveoli, small bronchi, and small blood vessels.[29] In acute and subacute cases, physical examination usually reveals dry crackles.[28] At least 5% of cases include pulmonary arterial hypertension.[28][30] The upper respiratory tract (including the larynx, pharynx, and sinuses) may be affected, which occurs in between 5 and 10% of cases.[31]
The four stages of pulmonary involvement are based on radiological stage of the disease, which is helpful in prognosis:[32]
- Stage I: bilateral hilar lymphadenopathy (BHL) alone
- Stage II: BHL with pulmonary infiltrates
- Stage III: pulmonary infiltrates without BHL
- Stage IV: fibrosis
Use of the Scadding scale only provides general information regarding the prognosis of the pulmonary disease over time. Caution is recommended, as it only shows a general relation with physiological markers of the disease and the variation is such that it has limited applicability in individual assessments, including treatment decisions.[12]
Skin
Sarcoidosis involves the skin in between 9 and 37% of cases and is more common in African Americans than in European Americans.[28] The skin is the second-most commonly affected organ after the lungs.[33] The most common lesions are erythema nodosum, plaques, maculopapular eruptions, subcutaneous nodules, and lupus pernio.[33] Treatment is not required, since the lesions usually resolve spontaneously in 2–4 weeks. Although it may be disfiguring, cutaneous sarcoidosis rarely causes major problems.[28][34][35] Sarcoidosis of the scalp presents with diffuse or patchy hair loss.[36][37]
Heart
Histologically, sarcoidosis of the heart is an active granulomatous inflammation surrounded by reactive oedema. The distribution of affected areas is patchy with localised enlargement of heart muscles. This causes scarring and remodelling of the heart, which leads to dilatation of heart cavities and thinning of heart muscles. As the situation progresses, it leads to aneurysm of heart chambers. When the distribution is diffuse, there would be dilatation of both ventricles of the heart, causing heart failure and arrhythmia. When the conduction system in the intraventricular septum is affected, it would lead to heart block, ventricular tachycardia and ventricular arrhythmia, causing sudden death. Nevertheless, the involvement of pericardium and heart valves are uncommon.[38]
The frequency of cardiac involvement varies and is significantly influenced by race; in Japan, more than 25% of those with sarcoidosis have symptomatic cardiac involvement, whereas in the US and Europe, only about 5% of cases present with cardiac involvement.[28] Autopsy studies in the US have revealed a frequency of cardiac involvement of about 20–30%, whereas autopsy studies in Japan have shown a frequency of 60%.[22] The presentation of cardiac sarcoidosis can range from asymptomatic conduction abnormalities to fatal ventricular arrhythmia.[39][40]
Conduction abnormalities are the most common cardiac manifestations of sarcoidosis in humans and can include complete heart block.[41] Second to conduction abnormalities, in frequency, are ventricular arrhythmias, which occurs in about 23% of cases with cardiac involvement.[41] Sudden cardiac death, either due to ventricular arrhythmias or complete heart block is a rare complication of cardiac sarcoidosis.[42][43] Cardiac sarcoidosis can cause fibrosis, granuloma formation, or the accumulation of fluid in the interstitium of the heart, or a combination of the former two.[44][45] Cardiac sarcoidosis may also cause congestive heart failure when granulomas cause myocardial fibrosis and scarring.[46] Congestive heart failure affects 25-75% of those with cardiac sarcoidosis. Diabetes mellitus and sarcoidosis-related arrhythmias are believed to be strong risk factors of heart failure in sarcoidosis.[47] A small (20-40%) increased risk of acute myocardial infarction has also been described.[48] Pulmonary arterial hypertension occurs by two mechanisms in cardiac sarcoidosis: reduced left heart function due to granulomas weakening the heart muscle or from impaired blood flow.[49]
Eye
Eye involvement occurs in about 10–90% of cases.[22] Manifestations in the eye include uveitis, uveoparotitis, and retinal inflammation, which may result in loss of visual acuity or blindness.[50][51] The most common ophthalmologic manifestation of sarcoidosis is uveitis.[22][52][53] The combination of anterior uveitis, parotitis, VII cranial nerve paralysis and fever is called uveoparotid fever or Heerfordt syndrome (D86.8). Development of scleral nodule associated with sarcoidosis has been observed.[54]
Nervous system
Any of the components of the nervous system can be involved.[55] Sarcoidosis affecting the nervous system is known as neurosarcoidosis.[55] Cranial nerves are most commonly affected, accounting for about 5–30% of neurosarcoidosis cases, and peripheral facial nerve palsy, often bilateral, is the most common neurological manifestation of sarcoidosis.[55][56][57] It occurs suddenly and is usually transient. The central nervous system involvement is present in 10–25% of sarcoidosis cases.[31] Other common manifestations of neurosarcoidosis include optic nerve dysfunction, papilledema, palate dysfunction, neuroendocrine changes, hearing abnormalities, hypothalamic and pituitary abnormalities, chronic meningitis, and peripheral neuropathy.[28] Myelopathy, that is spinal cord involvement, occurs in about 16–43% of neurosarcoidosis cases and is often associated with the poorest prognosis of the neurosarcoidosis subtypes.[55] Whereas facial nerve palsies and acute meningitis due to sarcoidosis tend to have the most favourable prognosis,[55] another common finding in sarcoidosis with neurological involvement is autonomic or sensory small-fiber neuropathy.[58][59] Neuroendocrine sarcoidosis accounts for about 5–10% of neurosarcoidosis cases and can lead to diabetes insipidus, changes in menstrual cycle and hypothalamic dysfunction.[55][57] The latter can lead to changes in body temperature, mood, and prolactin (see the endocrine and exocrine section for details).[55]
Endocrine and exocrine
Prolactin is frequently increased in sarcoidosis, between 3 and 32% of cases have hyperprolactinemia[60] this frequently leads to amenorrhea, galactorrhea, or nonpuerperal mastitis in women. It also frequently causes an increase in 1,25-dihydroxy vitamin D, the active metabolite of vitamin D, which is usually hydroxylated within the kidney, but in sarcoidosis patients, hydroxylation of vitamin D can occur outside the kidneys, namely inside the immune cells found in the granulomas the condition produces. 1,25-dihydroxy vitamin D is the main cause for hypercalcemia in sarcoidosis and is overproduced by sarcoid granulomata. Gamma-interferon produced by activated lymphocytes and macrophages plays a major role in the synthesis of 1 alpha, 25(OH)2D3.[61] Hypercalciuria (excessive secretion of calcium in one's urine) and hypercalcemia (an excessively high amount of calcium in the blood) are seen in <10% of individuals and likely results from the increased 1,25-dihydroxy vitamin D production.[62]
Thyroid dysfunction is seen in 4.2–4.6% of cases.[63][64]
Parotid enlargement occurs in about 5–10% of cases.[19] Bilateral involvement is the rule. The gland is usually not tender, but firm and smooth. Dry mouth can occur; other exocrine glands are affected only rarely.[28] The eyes, their glands, or the parotid glands are affected in 20–50% of cases.[65]
Gastrointestinal and genitourinary
Symptomatic gastrointestinal (GI) involvement occurs in less than 1% of cases (if one excludes the liver), and most commonly the stomach is affected, although the small or large intestine may also be affected in a small portion of cases.[19][66] Studies at autopsy have revealed GI involvement in less than 10% of people.[57] These cases would likely mimic Crohn's disease, which is a more commonly intestine-affecting granulomatous disease.[19] About 1–3% of people have evidence of pancreatic involvement at autopsy.[57] Symptomatic kidney involvement occurs in just 0.7% of cases, although evidence of kidney involvement at autopsy has been reported in up to 22% of people and occurs exclusively in cases of chronic disease.[19][22][57] Symptomatic kidney involvement is usually nephrocalcinosis, although granulomatous interstitial nephritis that presents with reduced creatinine clearance and little proteinuria is a close second.[19][57] Less commonly, the epididymis, testicles, prostate, ovaries, fallopian tubes, uterus, or the vulva may be affected, the latter may cause vulva itchiness.[22][67][68] Testicular involvement has been reported in about 5% of people at autopsy.[57][68] In males, sarcoidosis may lead to infertility.[68]
Around 70% of people have granulomas in their livers, although only in about 20–30% of cases, liver function test anomalies reflecting this fact are seen.[20][28] About 5–15% of patients exhibit hepatomegaly.[22] Only 5–30% of cases of liver involvement are symptomatic.[69] Usually, these changes reflect a cholestatic pattern and include raised levels of alkaline phosphatase (which is the most common liver function test anomaly seen in those with sarcoidosis), while bilirubin and aminotransferases are only mildly elevated. Jaundice is rare.[19][28]
Blood
Abnormal blood tests are frequent, accounting for over 50% of cases, but are not diagnostic.[28][31] Lymphopenia is the most common blood anomaly in sarcoidosis.[28] Anemia occurs in about 20% of people with sarcoidosis.[28] Leukopenia is less common and occurs in even fewer cases but is rarely severe.[28] Thrombocytopenia and hemolytic anemia are fairly rare.[19] In the absence of splenomegaly, leukopenia may reflect bone marrow involvement, but the most common mechanism is a redistribution of blood T cells to sites of disease.[70] Other nonspecific findings include monocytosis, occurring in the majority of sarcoidosis cases,[71] increased hepatic enzymes or alkaline phosphatase. People with sarcoidosis often have immunologic anomalies like allergies to test antigens such as Candida or purified protein derivative.[65] Polyclonal hypergammaglobulinemia is also a fairly common immunologic anomaly seen in sarcoidosis.[65]
Lymphadenopathy (swollen glands) is common in sarcoidosis and occurs in 15% of cases.[23] Intrathoracic nodes are enlarged in 75 to 90% of all people; usually this involves the hilar nodes, but the paratracheal nodes are commonly involved. Peripheral lymphadenopathy is very common, particularly involving the cervical (the most common head and neck manifestation of the disease), axillary, epitrochlear, and inguinal nodes.[72] Approximately 75% of cases show microscopic involvement of the spleen, although only in about 5–10% of cases does splenomegaly appear.[19][65]
Bone, joints, and muscles
Sarcoidosis can be involved with the joints, bones, and muscles. This causes a wide variety of musculoskeletal complaints that act through different mechanisms.[73] About 5–15% of cases affect the bones, joints, or muscles.[31]
Arthritic syndromes can be categorized as acute or chronic.[73] Sarcoidosis patients with acute arthritis often also have bilateral hilar lymphadenopathy and erythema nodosum. These three associated syndromes often occur together in Löfgren syndrome.[73] The arthritis symptoms of Löfgren syndrome occur most frequently in the ankles, followed by the knees, wrists, elbows, and metacarpophalangeal joints.[73] Usually, true arthritis is not present, but instead, periarthritis appears as a swelling in the soft tissue around the joints that can be seen by ultrasonographic methods.[73] These joint symptoms tend to precede or occur at the same time as erythema nodosum develops.[73] Even when erythema nodosum is absent, it is believed that the combination of hilar lymphadenopathy and ankle periarthritis can be considered as a variant of Löfgren syndrome.[73] Enthesitis also occurs in about one-third of patients with acute sarcoid arthritis, mainly affecting the Achilles tendon and heels.[73] Soft-tissue swelling of the ankles can be prominent, and biopsy of this soft tissue reveals no granulomas, but does show panniculitis similar to erythema nodosum.[73]
Chronic sarcoid arthritis usually occurs in the setting of more diffuse organ involvement.[73] The ankles, knees, wrists, elbows, and hands may all be affected in the chronic form and often this presents itself in a polyarticular pattern.[73] Dactylitis similar to that seen in psoriatic arthritis, that is associated with pain, swelling, overlying skin erythema, and underlying bony changes may also occur.[73] Development of Jaccoud arthropathy (a nonerosive deformity) is very rarely seen.[73]
Bone involvement in sarcoidosis has been reported in 1–13% of cases.[57] The most frequent sites of involvement are the hands and feet, whereas the spine is less commonly affected.[73] Half of the patients with bony lesions experience pain and stiffness, whereas the other half remain asymptomatic.[73] Periostitis is rarely seen in sarcoidosis and has been found to present itself at the femoral bone.[74][75]
Cause
The exact cause of sarcoidosis is not known.[2] The current working hypothesis is, in genetically susceptible individuals, sarcoidosis is caused through alteration to the immune response after exposure to an environmental, occupational, or infectious agent.[76] Some cases may be caused by treatment with tumor necrosis factor (TNF) inhibitors like etanercept.[77]
Genetics
The heritability of sarcoidosis varies according to ethnicity. About 20% of African Americans with sarcoidosis have a family member with the condition, whereas the same figure for European Americans is about 5%. Additionally, in African Americans, who seem to experience more severe and chronic disease, siblings and parents of sarcoidosis cases have about a 2.5-fold increased risk for developing the disease.[26] In Swedish individuals heritability was found to be 39%.[78] In this group, if a first-degree family member was affected, a person has a four-fold greater risk of being affected.[78]
Investigations of genetic susceptibility yielded many candidate genes, but only few were confirmed by further investigations and no reliable genetic markers are known. Currently, the most interesting candidate gene is BTNL2; several HLA-DR risk alleles are also being investigated.[79][80] In persistent sarcoidosis, the HLA haplotype HLA-B7-DR15 is either cooperating in disease or another gene between these two loci is associated. In nonpersistent disease, a strong genetic association exists with HLA DR3-DQ2.[81][82] Cardiac sarcoid has been connected to tumor necrosis factor alpha (TNFA) variants.[83]
Infectious agents
Several infectious agents appear to be significantly associated with sarcoidosis, but none of the known associations is specific enough to suggest a direct causative role.[84] The major implicated infectious agents include: mycobacteria, fungi, borrelia, and rickettsia.[85] A meta-analysis investigating the role of mycobacteria in sarcoidosis found it was present in 26.4% of cases, but they also detected a possible publication bias, so the results need further confirmation.[86][87] Mycobacterium tuberculosis catalase-peroxidase has been identified as a possible antigen catalyst of sarcoidosis.[88] The disease has also been reported by transmission via organ transplants.[89] A large epidemiological study found little evidence that infectious diseases spanning years before sarcoidosis diagnosis could confer measurable risks for sarcoidosis diagnosis in the future.[90]
Autoimmune
Association of autoimmune disorders has been frequently observed. The exact mechanism of this relation is not known, but some evidence supports the hypothesis that this is a consequence of Th1 lymphokine prevalence.[63][91] Tests of delayed cutaneous hypersensitivity have been used to measure progression.[92]
Pathophysiology
Granulomatous inflammation is characterized primarily by the accumulation of macrophages and activated T-lymphocytes, with increased production of key inflammatory mediators, tumor necrosis factor alpha (TNF), interferon gamma, interleukin 2 (IL-2), IL-8, IL-10, IL-12, IL-18, IL-23 and transforming growth factor beta (TGF-β), indicative of a T helper cell-mediated immune response.[85][93] Sarcoidosis has paradoxical effects on inflammatory processes; it is characterized by increased macrophage and CD4 helper T-cell activation, resulting in accelerated inflammation, but immune response to antigen challenges such as tuberculin is suppressed. This paradoxic state of simultaneous hyper- and hypoactivity is suggestive of a state of anergy. The anergy may also be responsible for the increased risk of infections and cancer.[citation needed] The regulatory T-lymphocytes in the periphery of sarcoid granulomas appear to suppress IL-2 secretion, which is hypothesized to cause the state of anergy by preventing antigen-specific memory responses.[94]
While TNF is widely believed to play an important role in the formation of granulomas (this is further supported by the finding that in animal models of mycobacterial granuloma formation inhibition of either TNF or IFN-γ production inhibits granuloma formation), sarcoidosis can and does still develop in those being treated with TNF antagonists like etanercept.[95] B cells also likely play a role in the pathophysiology of sarcoidosis.[26] Serum levels of soluble human leukocyte antigen (HLA) class I antigens and angiotensin converting enzyme (ACE) are higher in people with sarcoidosis.[26] Likewise the ratio of CD4/CD8 T cells in bronchoalveolar lavage is usually higher in people with pulmonary sarcoidosis (usually >3.5), although it can be normal or even abnormally low in some cases.[26] Serum ACE levels have been found to usually correlate with total granuloma load.[85]
Cases of sarcoidosis have also been reported as part of the immune reconstitution syndrome of HIV, that is, when people receive treatment for HIV, their immune system rebounds and the result is that it starts to attack the antigens of opportunistic infections caught prior to said rebound and the resulting immune response starts to damage healthy tissue.[93]
Histopathology
Sarcoidosis is characterized by the formation of non-necrotizing ("non-caseating") granulomas in various organs and tissues.[96] Giant cells, specifically Langhans giant cells, are often seen in sarcoidosis.[97] Schaumann bodies seen in sarcoidosis are calcium and protein inclusions inside of giant cells as part of a granuloma.[98] Asteroid bodies can be seen in sarcoidosis.[98] Hamazaki–Wesenberg bodies can be seen in lymph nodes and more rarely in lung biopsies with sarcoidosis and are inclusion bodies of lysosomes with protein, glycoprotein and iron.[99]
-
Sarcoidosis in a lymph node
-
Pulmonary sarcoidosis with granulomas with Langhans giant cells and asteroid bodies
-
Schaumann body in sarcoidosis
-
Asteroid body in sarcoidosis
-
Hamazaki–Wesenberg bodies in sarcoidosis in lymph node
Diagnosis
Diagnosis of sarcoidosis is a matter of exclusion, as there is no specific test for the condition. To exclude sarcoidosis in a case presenting with pulmonary symptoms might involve a chest radiograph, CT scan of chest, PET scan, CT-guided biopsy, mediastinoscopy, open lung biopsy, bronchoscopy with biopsy, endobronchial ultrasound, and endoscopic ultrasound with fine-needle aspiration of mediastinal lymph nodes (EBUS FNA). Tissue from biopsy of lymph nodes is subjected to both flow cytometry to rule out cancer and special stains (acid fast bacilli stain and Gömöri methenamine silver stain) to rule out microorganisms and fungi.[100][101][12][102]
Serum markers of sarcoidosis, include: serum amyloid A, soluble interleukin-2 receptor, lysozyme, angiotensin converting enzyme, and the glycoprotein KL-6.[103] Angiotensin-converting enzyme blood levels are used in the monitoring of sarcoidosis.[103] A bronchoalveolar lavage can show an elevated (of at least 3.5) CD4/CD8 T cell ratio, which is indicative (but not proof) of pulmonary sarcoidosis.[26] In at least one study the induced sputum ratio of CD4/CD8 and level of TNF was correlated to those in the lavage fluid.[103] A sarcoidosis-like lung disease called granulomatous–lymphocytic interstitial lung disease can be seen in patients with common variable immunodeficiency (CVID) and therefore serum antibody levels should be measured to exclude CVID.[citation needed]
Differential diagnosis includes metastatic disease, lymphoma, septic emboli, rheumatoid nodules, granulomatosis with polyangiitis, varicella infection, tuberculosis, and atypical infections, such as Mycobacterium avium complex, cytomegalovirus, and cryptococcus.[104] Sarcoidosis is confused most commonly with neoplastic diseases, such as lymphoma, or with disorders characterized also by a mononuclear cell granulomatous inflammatory process, such as the mycobacterial and fungal disorders.[28]
Chest radiograph changes are divided into four stages:[105]
- bihilar lymphadenopathy
- bihilar lymphadenopathy and reticulonodular infiltrates
- bilateral pulmonary infiltrates
- fibrocystic sarcoidosis typically with upward hilar retraction, cystic and bullous changes
Although people with stage 1 radiographs tend to have the acute or subacute, reversible form of the disease, those with stages 2 and 3 often have the chronic, progressive disease; these patterns do not represent consecutive "stages" of sarcoidosis. Thus, except for epidemiologic purposes, this categorization is mostly of historic interest.[28]
In sarcoidosis presenting in the Caucasian population, hilar adenopathy and erythema nodosum are the most common initial symptoms. In this population, a biopsy of the gastrocnemius muscle is a useful tool in correctly diagnosing the person. The presence of a noncaseating epithelioid granuloma in a gastrocnemius specimen is definitive evidence of sarcoidosis, as other tuberculoid and fungal diseases extremely rarely present histologically in this muscle.[106]
Cardiac magnetic resonance imaging (CMR) is one modality for diagnosing cardiac sarcoidosis. It has 78% specificity in diagnosing cardiac sarcoidosis.[38] Its T2-weighted imaging can detect acute inflammation. Meanwhile, late gadolinium contrast (LGE) can detect fibrosis or scar. Lesions at the subpericardium and midwall enhancement of basal septum or inferolateral wall is strongly suggestive of sarcoidosis.[38] MRI can also follow up on the treatment efficacy of corticosteroids and prognosis of cardiac sarcoidosis.[107]
PET scan is able to quantify disease activity which cannot be performed by CMR.[108]
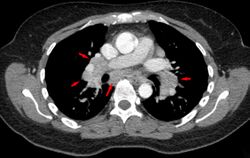
-
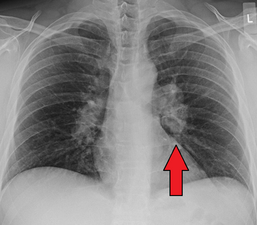
Hilar adenopathy especially on the person's left (AP CXR)
-
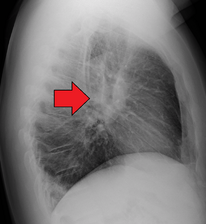
Hilar adenopathy especially on the person's left (lateral CXR)
-
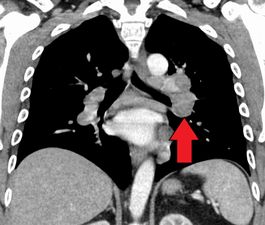
Hilar adenopathy especially on the person's left (coronal CT)
-
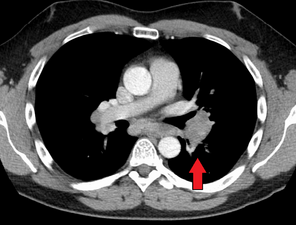
Hilar adenopathy especially on the person's left (transverse CT)
Classification
Sarcoidosis may be divided into the following types:[36]
- Annular sarcoidosis
- Erythrodermic sarcoidosis
- Ichthyosiform sarcoidosis
- Hypopigmented sarcoidosis
- Löfgren syndrome
- Lupus pernio
- Morpheaform sarcoidosis
- Mucosal sarcoidosis
- Neurosarcoidosis
- Papular sarcoid
- Scar sarcoid
- Subcutaneous sarcoidosis
- Systemic sarcoidosis
- Ulcerative sarcoidosis
Treatment
Treatments for sarcoidosis vary greatly depending on the patient.[109] At least half of patients require no systemic therapy.[110] Most people (>75%) only require symptomatic treatment with nonsteroidal anti-inflammatory drugs (NSAIDs) like ibuprofen or aspirin.[111] For those presenting with lung symptoms, unless the respiratory impairment is devastating, active pulmonary sarcoidosis is observed usually without therapy for two to three months; if the inflammation does not subside spontaneously, therapy is instituted.[28]
Major categories of drug interventions include glucocorticoids, antimetabolites, biologic agents especially monoclonal anti-tumor necrosis factor antibodies.[110] Investigational treatments include specific antibiotic combinations and mesenchymal stem cells.[110] If drug intervention is indicated, a step-wise approach is often used to explore alternatives in order of increasing side effects and to monitor potentially toxic effects.[110]
Corticosteroids, most commonly prednisone or prednisolone, have been the standard treatment for many years.[19] In some people, this treatment can slow or reverse the course of the disease, but other people do not respond to steroid therapy. The use of corticosteroids in mild disease is controversial because in many cases the disease remits spontaneously.[112][113]
Antimetabolites
Antimetabolites, also categorized as steroid-sparing agents, such as azathioprine, methotrexate, mycophenolic acid, and leflunomide[114][115] are often used as alternatives to corticosteroids.[19][116] Of these, methotrexate is most widely used and studied.[116][117] Methotrexate is considered a first-line treatment in neurosarcoidosis, often in conjunction with corticosteroids.[55][116] Long-term treatment with methotrexate is associated with liver damage in about 10% of people and hence may be a significant concern in people with liver involvement and requires regular liver function test monitoring.[19] Methotrexate can also lead to pulmonary toxicity (lung damage), although this is fairly uncommon and more commonly it can confound the leukopenia caused by sarcoidosis.[19] Due to these safety concerns it is often recommended that methotrexate is combined with folic acid in order to prevent toxicity.[19] Azathioprine treatment can also lead to liver damage.[117] However, the risk of infection appears to be about 40% lower in those treated with methotrexate instead of azathioprine.[118] Leflunomide is being used as a replacement for methotrexate, possibly due to its purportedly lower rate of pulmonary toxicity.[117] Mycophenolic acid has been used successfully in uveal sarcoidosis,[119] neurosarcoidosis (especially CNS sarcoidosis; minimally effective in sarcoidosis myopathy),[120] and pulmonary sarcoidosis.[121][122]
Immunosuppressants
As the granulomas are caused by collections of immune system cells, particularly T cells, there has been some success using immunosuppressants (like cyclophosphamide, cladribine,[123] chlorambucil, and cyclosporine), immunomodulatory (pentoxifylline and thalidomide), and anti-tumor necrosis factor treatment[124][125] (such as infliximab, etanercept, golimumab, and adalimumab).[17][126][127]
In a clinical trial cyclosporine added to prednisone treatment failed to demonstrate any significant benefit over prednisone alone in people with pulmonary sarcoidosis, although there was evidence of increased toxicity from the addition of cyclosporine to the steroid treatment including infections, malignancies (cancers), hypertension, and kidney dysfunction.[117] Likewise chlorambucil and cyclophosphamide are seldom used in the treatment of sarcoidosis due to their high degree of toxicity, especially their potential for causing malignancies.[128] Infliximab has been used successfully to treat pulmonary sarcoidosis in clinical trials in a number of cases.[117] Etanercept, on the other hand, has failed to demonstrate any significant efficacy in people with uveal sarcoidosis in a couple of clinical trials.[117] Likewise golimumab has failed to show any benefit in those with pulmonary sarcoidosis.[117] One clinical trial of adalimumab found treatment response in about half of subjects, which is similar to that seen with infliximab, but as adalimumab has better tolerability profile it may be preferred over infliximab.[117]
Specific organ treatments
Ursodeoxycholic acid has been used successfully as a treatment for cases with liver involvement.[129] Thalidomide has also been tried successfully as a treatment for treatment-resistant lupus pernio in a clinical trial, which may stem from its anti-TNF activity, although it failed to exhibit any efficacy in a pulmonary sarcoidosis clinical trial.[93][126] Cutaneous disease may be successfully managed with antimalarials (such as chloroquine and hydroxychloroquine) and the tetracycline antibiotic, minocycline.[28][126] Antimalarials have also demonstrated efficacy in treating sarcoidosis-induced hypercalcemia and neurosarcoidosis.[19] Long-term use of antimalarials is limited, however, by their potential to cause irreversible blindness and hence the need for regular ophthalmologic screening.[128] This toxicity is usually less of a problem with hydroxychloroquine than with chloroquine, although hydroxychloroquine can disturb the glucose homeostasis.[128]
Recently selective phosphodiesterase 4 (PDE4) inhibitors like apremilast (a thalidomide derivative), roflumilast, and the less subtype-selective PDE4 inhibitor, pentoxifylline, have been tried as a treatment for sarcoidosis, with successful results being obtained with apremilast in cutaneous sarcoidosis in a small open-label study.[130][131] Pentoxifylline has been used successfully to treat acute disease although its use is greatly limited by its gastrointestinal toxicity (mostly nausea, vomiting, and diarrhea).[115][117][128] Case reports have supported the efficacy of rituximab, an anti-CD20 monoclonal antibody and a clinical trial investigating atorvastatin as a treatment for sarcoidosis is under-way.[132][133] ACE inhibitors have been reported to cause remission in cutaneous sarcoidosis and improvement in pulmonary sarcoidosis, including improvement in pulmonary function, remodeling of lung parenchyma and prevention of pulmonary fibrosis in separate case series'.[134][135] Nicotine patches have been found to possess anti-inflammatory effects in sarcoidosis patients, although whether they had disease-modifying effects requires further investigation.[136] Antimycobacterial treatment (drugs that kill off mycobacteria, the causative agents behind tuberculosis and leprosy) has also proven itself effective in treating chronic cutaneous (that is, it affects the skin) sarcoidosis in one clinical trial.[137] Quercetin has also been tried as a treatment for pulmonary sarcoidosis with some early success in one small trial.[138]
Because of its uncommon nature, the treatment of male reproductive tract sarcoidosis is controversial. Since the differential diagnosis includes testicular cancer, some recommend orchiectomy, even if evidence of sarcoidosis in other organs is present. In the newer approach, testicular, epididymal biopsy and resection of the largest lesion has been proposed.[68]
Symptoms
People with sarcoidosis may have a range of symptoms that do not correspond with objective physical evidence of disease but that still decrease quality of life.[139]
Physical therapy, rehabilitation, and counseling can help avoid deconditioning,[139]:733 and improve social participation, psychological well-being, and activity levels. Key aspects are avoiding exercise intolerance and muscle weakness.[139]:734
Low or moderate-intensity physical training has been shown to improve fatigue, psychological health, and physical functioning in people sarcoidosis without adverse effects.[140][141] Inspiratory muscle training has also decreased severe fatigue perception in subjects with early stages of sarcoidosis, as well as improving functional and maximal exercise capacity and respiratory muscle strength.[142] The duration, frequency, and physical intensity of exercise needs to accommodate impairments such as joint pain, muscle pain, and fatigue.[139]:734[141][143]
Neurostimulants such as methylphenidate and modafinil have shown some effectiveness as an adjunct for treatment of sarcoidosis fatigue.[139]:733[144]
Treatments for symptomatic neuropathic pain in sarcoidosis patients is similar to that for other causes, and include antidepressants, anticonvulsants and prolonged-release opioids, however, only 30 to 60% of patients experience limited pain relief.[139]:733
Prognosis
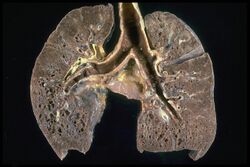
The disease can remit spontaneously or become chronic, with exacerbations and remissions. In some cases, it can progress to pulmonary fibrosis and death. In benign cases, remission can occur in 24 to 36 months without treatment but regular follow ups are required. Some cases, however, may persist several decades.[19] Two-thirds of people with the condition achieve a remission within 10 years of the diagnosis.[145] When the heart is involved, the prognosis is generally less favourable, though corticosteroids appear effective in improving AV conduction.[146][147] The prognosis tends to be less favourable in African Americans than in white Americans.[26] In a Swedish population-based analysis, the majority of cases who did not have severe disease at diagnosis had comparable mortality to the general population.[148] The risk for premature death was markedly (2.3-fold) increased compared to the general population for a smaller group of cases with severe disease at diagnosis.[148] Serious infections, sometimes multiple during the course of disease, and heart failure might contribute to the higher risk of early death in some patients with sarcoidosis.[149][150]
Some 1990s studies indicated that people with sarcoidosis appear to be at significantly increased risk for cancer, in particular lung cancer, lymphomas,[151] and cancer in other organs known to be affected in sarcoidosis.[152][153] In sarcoidosis-lymphoma syndrome, sarcoidosis is followed by the development of a lymphoproliferative disorder such as non-Hodgkin lymphoma.[154] This may be attributed to the underlying immunological abnormalities that occur during the sarcoidosis disease process.[155] Sarcoidosis can also follow cancer[156][157] or occur concurrently with cancer.[158][159] There have been reports of hairy cell leukemia,[160] acute myeloid leukemia,[161] and acute myeloblastic leukemia[162] associated with sarcoidosis. Sometimes, sarcoidosis, even untreated, can be complicated by opportunistic infections although these are rare.[163][164][149]
Epidemiology
Sarcoidosis most commonly affects young adults of both sexes, although studies have reported more cases in females. Incidence is highest for individuals younger than 40 and peaks in the age-group from 20 to 29 years; a second peak is observed for women over 50.[19][146]
Sarcoidosis occurs throughout the world in all races with an average incidence of 16.5 per 100,000 in men and 19 per 100,000 in women. The disease is most common in Northern European countries and the highest annual incidence of 60 per 100,000 is found in Sweden and Iceland. In the United Kingdom the prevalence is 16 in 100,000.[165] In the United States, sarcoidosis is more common in people of African descent than Caucasians, with annual incidence reported as 35.5 and 10.9 per 100,000, respectively.[166] Sarcoidosis is less commonly reported in South America, Spain, India, Canada, and the Philippines. There may be a higher susceptibility to sarcoidosis in those with celiac disease. An association between the two disorders has been suggested.[167]
There also has been a seasonal clustering observed in sarcoidosis-affected individuals.[168] In Greece about 70% of diagnoses occur between March and May every year, in Spain about 50% of diagnoses occur between April and June, and in Japan it is mostly diagnosed during June and July.[168]
The differing incidence across the world may be at least partially attributable to the lack of screening programs in certain regions of the world, and the overshadowing presence of other granulomatous diseases, such as tuberculosis, that may interfere with the diagnosis of sarcoidosis where they are prevalent.[146] There may also be differences in the severity of the disease between people of different ethnicities. Several studies suggest the presentation in people of African origin may be more severe and disseminated than for Caucasians, who are more likely to have asymptomatic disease.[70] Manifestation appears to be slightly different according to race and sex. Erythema nodosum is far more common in men than in women and in Caucasians than in other races. In Japanese people, ophthalmologic and cardiac involvement are more common than in other races.[19]
It is more common in certain occupations, namely firefighters, educators, military personnel, those who work in industries where pesticides are used, law enforcement, and healthcare personnel.[169] In the year after the September 11 attacks, the rate of sarcoidosis incidence went up four-fold (to 86 cases per 100,000).[31][169]
History
It was first described in 1877 by Dr. Jonathan Hutchinson, a dermatologist as a condition causing red, raised rashes on the face, arms, and hands.[15] In 1889 the term lupus pernio was coined by Dr. Ernest Besnier, another dermatologist.[170] Later in 1892 lupus pernio's histology was defined.[170] In 1902 bone involvement was first described by a group of three doctors.[170] Between 1909 and 1910 uveitis in sarcoidosis was first described, and later in 1915 it was emphasised, by Dr. Jörgen Nielsen Schaumann, that it was a systemic condition.[170] This same year lung involvement was also described.[170] In 1937 uveoparotid fever was first described and likewise in 1941 Löfgren syndrome was first described.[170] In 1958 the first international conference on sarcoidosis was called in London, likewise the first USA sarcoidosis conference occurred in Washington, D.C., in the year 1961.[170] It has also been called Besnier–Boeck disease or Besnier–Boeck–Schaumann disease.[171]
Etymology
The word "sarcoidosis" comes from Greek [σάρκο-] sarco- meaning "flesh", the suffix -(e)ido (from the Greek εἶδος -eidos [usually omitting the initial e in English as the diphthong epsilon-iota in Classic Greek stands for a long "i" = English ee]) meaning "type", " resembles" or "like", and -sis, a common suffix in Greek meaning "condition". Thus the whole word means "a condition that resembles crude flesh". The first cases of sarcoïdosis, which were recognised as a new pathological entity, in Scandinavia, at the end of the 19th century exhibited skin nodules resembling cutaneous sarcomas, hence the name initially given.[citation needed]
Society and culture
The World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) is an organisation of physicians involved in the diagnosis and treatment of sarcoidosis and related conditions.[172] WASOG publishes the journal Sarcoidosis, Vasculitis and Diffuse Lung Diseases.[173] Additionally, the Foundation for Sarcoidosis Research (FSR) is devoted to supporting research into sarcoidosis and its possible treatments.[174]
There have been concerns that World Trade Center rescue workers are at a heightened risk for sarcoidosis.[175][176]
Comedian and actor Bernie Mac had sarcoidosis. In 2005, he mentioned that the disease was in remission.[177] His death on August 9, 2008, was caused by complications from pneumonia, though Mac's agent states the sarcoidosis was not related to his fatal pneumonia.[178]
Karen "Duff" Duffy, MTV personality and actress, was diagnosed with neurosarcoidosis in 1995.[179]
American football player Reggie White died in 2004, with pulmonary and cardiac sarcoidosis being contributing factors to his fatal heart arrhythmia.[180]
Singer Sean Levert died in 2008 of sarcoidosis complications.[181]
Manning Marable, a professor of public policy at Columbia University, died of pneumonia in 2011, less than a year after undergoing a double lung transplant following a diagnosis of sarcoidosis. In 2012, he was posthumously awarded a Pulitzer Prize in history for his biography "Malcolm X: A Life of Reinvention."
Joseph Rago, Pulitzer Prize-winning writer known for his work at The Wall Street Journal, died of sarcoidosis complications in 2017.[182]
Several historical figures are suspected of having sarcoidosis. In a 2014 letter to the British medical journal The Lancet, it was suggested that the French Revolution leader Maximilien Robespierre may have had sarcoidosis, causing him impairment during his time as head of the Reign of Terror.[183] The symptoms associated with Ludwig van Beethoven's 1827 death have been described as possibly consistent with sarcoidosis.[184] Author Robert Louis Stevenson (1850–1894) had a history of chronic coughs and chest complaints, and sarcoidosis has been suggested as a diagnosis.[185]
Pregnancy
Sarcoidosis generally does not prevent successful pregnancy and delivery; the increase in estrogen levels during pregnancy may even have a slightly beneficial immunomodulatory effect. In most cases, the course of the disease is unaffected by pregnancy, with improvement in a few cases and worsening of symptoms in very few cases, although it is worth noting that a number of the immunosuppressants (such as methotrexate, cyclophosphamide) used in corticosteroid-refractory sarcoidosis are known teratogens.[186] Increased risks associated with sarcoidosis ranging from 30 to 70% have been reported for preeclampsia/eclampsia, cesarian or preterm delivery as well as (non-cardiac) birth defects in first singleton pregnancies.[187] In absolute numbers, birth defects and other complications such as maternal death, cardiac arrest, placental abruption or venous thromboembolism are extremely rare in sarcoidosis pregnancies.[187]
References
- ↑ (in en) Elsevier's Dictionary of Medicine and Biology: in English, Greek, German, Italian and Latin. Elsevier. 2005. p. 1454. ISBN 978-0-08-046012-3. https://books.google.com/books?id=lCKRIT-7X6UC&pg=PA1454.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 "What Is Sarcoidosis?". June 14, 2013. http://www.nhlbi.nih.gov/health/health-topics/topics/sarc.
- ↑ 3.0 3.1 3.2 3.3 "What Are the Signs and Symptoms of Sarcoidosis?". June 14, 2013. http://www.nhlbi.nih.gov/health/health-topics/topics/sarc/signs.
- ↑ 4.0 4.1 4.2 4.3 4.4 "Who Is at Risk for Sarcoidosis?". NHLBI. June 14, 2013. http://www.nhlbi.nih.gov/health/health-topics/topics/sarc/atrisk.
- ↑ 5.0 5.1 5.2 5.3 5.4 "Treatment of Sarcoidosis". Clinics in Chest Medicine 36 (4): 751–67. December 2015. doi:10.1016/j.ccm.2015.08.015. PMID 26593147.
- ↑ 6.0 6.1 6.2 6.3 "The Diagnosis of Sarcoidosis". Clinics in Chest Medicine 36 (4): 585–602. December 2015. doi:10.1016/j.ccm.2015.08.003. PMID 26593135.
- ↑ Ferri's differential diagnosis: a practical guide to the differential diagnosis of symptoms, signs, and clinical disorders (2nd ed.). Philadelphia, PA: Elsevier/Mosby. 2010. p. Chapter S. ISBN 978-0-323-07699-9.
- ↑ 8.0 8.1 "Pulmonology meets rheumatology in sarcoidosis: a review on the therapeutic approach". Current Opinion in Rheumatology 26 (3): 276–84. May 2014. doi:10.1097/bor.0000000000000052. PMID 24614277.
- ↑ 9.0 9.1 9.2 "Corticosteroids in Sarcoidosis". Rheumatic Disease Clinics of North America 42 (1): 119–35, ix. February 2016. doi:10.1016/j.rdc.2015.08.012. PMID 26611555.
- ↑ 10.0 10.1 GBD 2015 Disease Injury Incidence Prevalence Collaborators (October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMID 27733282.
- ↑ 11.0 11.1 GBD 2015 Mortality Causes of Death Collaborators (October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet 388 (10053): 1459–1544. doi:10.1016/S0140-6736(16)31012-1. PMID 27733281.
- ↑ 12.0 12.1 12.2 "A concise review of pulmonary sarcoidosis". American Journal of Respiratory and Critical Care Medicine 183 (5): 573–81. March 2011. doi:10.1164/rccm.201006-0865CI. PMID 21037016.
- ↑ "What Causes Sarcoidosis?". June 14, 2013. http://www.nhlbi.nih.gov/health/health-topics/topics/sarc/causes.
- ↑ 14.0 14.1 "Sarcoidosis: a rheumatologist's perspective". Therapeutic Advances in Musculoskeletal Disease 7 (5): 196–205. October 2015. doi:10.1177/1759720x15591310. PMID 26425148.
- ↑ 15.0 15.1 "From Hutchinson to now: a historical glimpse". Current Opinion in Pulmonary Medicine 8 (5): 416–23. September 2002. doi:10.1097/00063198-200209000-00013. PMID 12172446. http://www.ildcare.eu/Downloads/artseninfo/History_of_sarcoidosis.pdf.
- ↑ "Lung Diseases: Sarcoidosis: Signs & Symptoms". National Heart, Lung, and Blood Institute. http://www.nhlbi.nih.gov/health/dci/Diseases/sarc/sar_signsandsymptoms.html.
- ↑ 17.0 17.1 "Sarcoidosis Clinical Presentation". Medscape Reference. WebMD. 6 February 2014. http://emedicine.medscape.com/article/301914-clinical#showall.
- ↑ de Kleijn, Willemien PE; De Vries, Jolanda; Lower, Elyse E; Elfferich, Marjon DP; Baughman, Robert P; Drent, Marjolein (September 2009). "Fatigue in sarcoidosis: a systematic review" (in en). Current Opinion in Pulmonary Medicine 15 (5): 499–506. doi:10.1097/MCP.0b013e32832d0403. ISSN 1070-5287. PMID 19458531. https://dx.doi.org/10.1097/MCP.0b013e32832d0403.
- ↑ 19.00 19.01 19.02 19.03 19.04 19.05 19.06 19.07 19.08 19.09 19.10 19.11 19.12 19.13 19.14 19.15 19.16 19.17 "Sarcoidosis". Orphanet Journal of Rare Diseases 2: 46. November 2007. doi:10.1186/1750-1172-2-46. PMID 18021432.
- ↑ 20.0 20.1 20.2 20.3 King, TE Jr. (March 2008). "Sarcoidosis: Interstitial Lung Diseases: Merck Manual Home Edition". The Merck Manual Home Edition. Merck Sharp & Dohme Corp. http://www.merckmanuals.com/home/lung_and_airway_disorders/interstitial_lung_diseases/sarcoidosis.html.
- ↑ "Rheumatologic manifestations of sarcoidosis". Seminars in Respiratory and Critical Care Medicine 31 (4): 463–73. August 2010. doi:10.1055/s-0030-1262214. PMID 20665396.
- ↑ 22.0 22.1 22.2 22.3 22.4 22.5 22.6 "Sarcoidosis: extrathoracic manifestations". Disease-a-Month 55 (11): 675–92. November 2009. doi:10.1016/j.disamonth.2009.05.002. PMID 19857642.
- ↑ 23.0 23.1 "Sarcoidosis". BMJ 339: b3206. August 2009. doi:10.1136/bmj.b3206. PMID 19717499.
- ↑ Holas, Pawel; Kowalski, Joachim; Dubaniewicz, Anna; Farnik, Małgorzata; Jarzemska, Agnieszka; Warzechowska, Marta Maskey-; Bielecki, Maximilian; Domagala-Kulawik, Joanna (2018-07-01). "Relationship of emotional distress and physical concerns with fatigue severity in sarcoidosis" (in en). Sarcoidosis Vasculitis and Diffuse Lung Disease 35 (2): 160–164. doi:10.36141/svdld.v35i2.6604. ISSN 2532-179X. PMID 32476897. PMC 7170092. https://www.mattioli1885journals.com/.
- ↑ "Sarcoidosis mimicking metastatic breast cancer". Clinical Breast Cancer 7 (10): 804–10. October 2007. doi:10.3816/CBC.2007.n.044. PMID 18021484.
- ↑ 26.0 26.1 26.2 26.3 26.4 26.5 26.6 "Sarcoidosis". Medscape Reference. WebMD. 6 February 2014. http://emedicine.medscape.com/article/301914-overview#showall.
- ↑ "Pulmonary manifestations of sarcoidosis". Presse Médicale 41 (6 Pt 2): e289–302. June 2012. doi:10.1016/j.lpm.2012.03.019. PMID 22579234.
- ↑ 28.00 28.01 28.02 28.03 28.04 28.05 28.06 28.07 28.08 28.09 28.10 28.11 28.12 28.13 28.14 28.15 28.16 28.17 Harrison's Principles of Internal Medicine (18th ed.). New York: McGraw-Hill Professional. 2011. ISBN 978-0-07174889-6.
- ↑ "Intrathoracic sarcoidosis". Disease-a-Month 55 (11): 661–74. November 2009. doi:10.1016/j.disamonth.2009.04.009. PMID 19857641.
- ↑ "Pulmonary hypertension complicating sarcoidosis". Presse Médicale 41 (6 Pt 2): e303–16. June 2012. doi:10.1016/j.lpm.2012.04.003. PMID 22608948.
- ↑ 31.0 31.1 31.2 31.3 31.4 "Sarcoidosis--scientific progress and clinical challenges". Nature Reviews. Rheumatology 7 (8): 457–67. July 2011. doi:10.1038/nrrheum.2011.93. PMID 21750528.
- ↑ Kumar and Clark, Clinical Medicine, 8th edition, p. 846.
- ↑ 33.0 33.1 "Skin manifestations of sarcoidosis". Presse Médicale 41 (6 Pt 2): e355–74. June 2012. doi:10.1016/j.lpm.2012.02.046. PMID 22579238.
- ↑ "Sarcoidosis: Are there differences in your skin of color patients?". Journal of the American Academy of Dermatology 66 (1): 121.e1–14. January 2012. doi:10.1016/j.jaad.2010.06.068. PMID 22000704.
- ↑ "Sarcoidosis of the skin: a review for the pulmonologist". Chest 136 (2): 583–596. August 2009. doi:10.1378/chest.08-1527. PMID 19666758.
- ↑ 36.0 36.1 Andrew's Diseases of the Skin: Clinical Dermatology (10th ed.). Philadelphia: Saunders Elsevier. 2006. pp. 708–711. ISBN 978-0808923510.
- ↑ "Sarcoidosis-induced alopecia". Dermatology Online Journal 18 (8): 4. August 2012. doi:10.5070/D30FP92370. PMID 22948054. http://escholarship.org/uc/item/0fp92370.
- ↑ 38.0 38.1 38.2 "Cardiac Sarcoidosis: A Review of Contemporary Challenges in Diagnosis and Treatment". The American Journal of the Medical Sciences 355 (2): 113–125. February 2018. doi:10.1016/j.amjms.2017.08.009. PMID 29406038.
- ↑ "HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis". Heart Rhythm 11 (7): 1305–23. July 2014. doi:10.1016/j.hrthm.2014.03.043. PMID 24819193.
- ↑ "Cardiac sarcoidosis". Heart 92 (2): 282–8. February 2006. doi:10.1136/hrt.2005.080481. PMID 16415205.
- ↑ 41.0 41.1 "Cardiac sarcoidosis: applications of imaging in diagnosis and directing treatment". Heart 97 (24): 2078–87. December 2011. doi:10.1136/hrt.2011.226076. PMID 22116891.
- ↑ "Myocardial sarcoidosis as a rare cause of sudden cardiac death". Forensic Science International 89 (3): 145–53. October 1997. doi:10.1016/S0379-0738(97)00106-0. PMID 9363623.
- ↑ "Myocardial sarcoidosis, bouts of ventricular tachycardia, psychiatric manifestations and sudden death. A case report". Journal of the National Medical Association 61 (4): 306–9. July 1969. PMID 5796402.
- ↑ "Cardiac sarcoidosis". Presse Médicale 41 (6 Pt 2): e317–30. June 2012. doi:10.1016/j.lpm.2012.04.002. PMID 22608949.
- ↑ "Cardiac sarcoidosis-state of the art review". Cardiovascular Diagnosis and Therapy 6 (1): 50–63. February 2016. doi:10.3978/j.issn.2223-3652.2015.12.13. PMID 26885492.
- ↑ "About Sarcoidosis" (in en). http://med.stanford.edu/sarcoidosis/about-.html.
- ↑ Rossides, Marios; Kullberg, Susanna; Grunewald, Johan; Eklund, Anders; Di Giuseppe, Daniela; Askling, Johan; Arkema, Elizabeth V (2021-05-21). "Risk and predictors of heart failure in sarcoidosis in a population-based cohort study from Sweden" (in en). Heart 108 (6): heartjnl–2021–319129. doi:10.1136/heartjnl-2021-319129. ISSN 1355-6037. PMID 34021039.
- ↑ Rossides, Marios; Kullberg, Susanna; Grunewald, Johan; Eklund, Anders; Di Giuseppe, Daniela; Askling, Johan; Arkema, Elizabeth V. (2021). "Risk of acute myocardial infarction in sarcoidosis: A population-based cohort study from Sweden" (in en). Respiratory Medicine 188: 106624. doi:10.1016/j.rmed.2021.106624. PMID 34583304.
- ↑ "Cardiac sarcoidosis: a comprehensive review". Archives of Medical Science 7 (4): 546–54. August 2011. doi:10.5114/aoms.2011.24118. PMID 22291785.
- ↑ "Ocular sarcoidosis". Presse Médicale 41 (6 Pt 2): e349–54. June 2012. doi:10.1016/j.lpm.2012.04.004. PMID 22595776.
- ↑ Rissardo, JamirPitton; Fornari Caprara, AnaLetícia (2019). "Ocular involvement of sarcoidosis" (in en). The Pan-American Journal of Ophthalmology 1 (1): 17. doi:10.4103/PAJO.PAJO_23_19. ISSN 2666-4909. http://www.thepajo.org/text.asp?2019/1/1/17/272219.
- ↑ "Sarcoidosis and uveitis". Autoimmunity Reviews 13 (8): 840–9. August 2014. doi:10.1016/j.autrev.2014.04.001. PMID 24704868.
- ↑ "Diagnosis of ocular sarcoidosis". Ocular Immunology and Inflammation 18 (6): 432–41. December 2010. doi:10.3109/09273948.2010.524344. PMID 21091056.
- ↑ "Scleral nodule associated with sarcoidosis". American Journal of Ophthalmology 136 (4): 752–4. October 2003. doi:10.1016/S0002-9394(03)00454-9. PMID 14516826.
- ↑ 55.0 55.1 55.2 55.3 55.4 55.5 55.6 55.7 "Neurosarcoidosis: Clinical manifestations, diagnosis and treatment". Presse Médicale 41 (6 Pt 2): e331–48. June 2012. doi:10.1016/j.lpm.2011.12.017. PMID 22595777.
- ↑ "Nerve granulomas and vasculitis in sarcoid peripheral neuropathy: a clinicopathological study of 11 patients". Brain 125 (Pt 2): 264–75. February 2002. doi:10.1093/brain/awf027. PMID 11844727.
- ↑ 57.0 57.1 57.2 57.3 57.4 57.5 57.6 57.7 "Extra-pulmonary manifestations of sarcoidosis". Clinical Radiology 67 (3): 263–76. March 2012. doi:10.1016/j.crad.2011.04.018. PMID 22094184.
- ↑ "Sarcoidosis and small-fiber neuropathy". Current Pain and Headache Reports 15 (3): 201–6. June 2011. doi:10.1007/s11916-011-0180-8. PMID 21298560.
- ↑ "Sarcoidosis and pain caused by small-fiber neuropathy". Pain Research and Treatment 2012: 1–6. 2012. doi:10.1155/2012/256024. PMID 23304492.
- ↑ "Endocrine and reproductive manifestations of sarcoidosis". QJM 96 (8): 553–61. August 2003. doi:10.1093/qjmed/hcg103. PMID 12897340.
- ↑ "Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D". The New England Journal of Medicine 305 (8): 440–3. August 1981. doi:10.1056/NEJM198108203050807. PMID 6894783.
- ↑ Rheumatology Diagnosis & Therapies (2nd ed.). Philadelphia: Lippincott Williams & Wilkins. 2005. p. 342.
- ↑ 63.0 63.1 "Prevalence of hypothyroidism and Graves disease in sarcoidosis". Chest 130 (2): 526–32. August 2006. doi:10.1378/chest.130.2.526. PMID 16899854.
- ↑ "Thyroid: an unusual hideout for sarcoidosis". Endocrine Practice 19 (2): e40–3. March–April 2013. doi:10.4158/EP12131.CR. PMID 23337134. http://aace.metapress.com/content/h35854138g847h80/fulltext.pdf.
- ↑ 65.0 65.1 65.2 65.3 Robbins and Cotran Pathologic Basis of disease. (7th ed.). Philadelphia, PA: Elsevier/Saunders. 2004. pp. 737–9. ISBN 978-0721601878.
- ↑ "Gastric sarcoidosis: a rare clinical presentation". Case Reports in Gastrointestinal Medicine 2013: 260704. 2013-01-01. doi:10.1155/2013/260704. PMID 24368949.
- ↑ "Vulvar sarcoidosis: case report and review of the literature". Journal of Cutaneous Medicine and Surgery 17 (4): 287–90. July–August 2013. doi:10.2310/7750.2012.12083. PMID 23815963.
- ↑ 68.0 68.1 68.2 68.3 "Testicular masses in a man with a plausible sarcoidosis". Indian Journal of Urology 27 (2): 269–71. April 2011. doi:10.4103/0970-1591.82848. PMID 21814320.
- ↑ "Therapeutic approach of hepatic sarcoidosis". Current Opinion in Pulmonary Medicine 18 (5): 472–82. September 2012. doi:10.1097/MCP.0b013e3283541626. PMID 22617809.
- ↑ 70.0 70.1 "Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999". American Journal of Respiratory and Critical Care Medicine 160 (2): 736–55. August 1999. doi:10.1164/ajrccm.160.2.ats4-99. PMID 10430755.
- ↑ "Immuno-cytological blood tests in cases of sarcoidosis". Sarcoidosis 3 (1): 52–9. March 1986. PMID 3033787.
- ↑ "Sarcoidal granuloma in cervical lymph nodes". Journal of the Chinese Medical Association 68 (7): 339–42. July 2005. doi:10.1016/S1726-4901(09)70172-8. PMID 16038376.
- ↑ 73.00 73.01 73.02 73.03 73.04 73.05 73.06 73.07 73.08 73.09 73.10 73.11 73.12 73.13 73.14 "Extrapulmonary manifestations of sarcoidosis". Rheumatic Disease Clinics of North America 39 (2): 277–97. May 2013. doi:10.1016/j.rdc.2013.02.007. PMID 23597964.
- ↑ "Granulomatous periostitis and tracheal involvement in sarcoidosis". Rheumatology 55 (1): 102. January 2016. doi:10.1093/rheumatology/kev319. PMID 26320137.
- ↑ "Sarcoidosis with palpable nodular myositis, periostitis and large-vessel vasculitis stimulating Takayasu's arteritis". Rheumatology 38 (3): 287–8. March 1999. doi:10.1093/rheumatology/38.3.287. PMID 10325674.
- ↑ "Lesson learned from ACCESS (A Case Controlled Etiologic Study of Sarcoidosis)". Proceedings of the American Thoracic Society 4 (5): 453–6. August 2007. doi:10.1513/pats.200607-138MS. PMID 17684288.
- ↑ "Sarcoidosis as an adverse effect of tumor necrosis factor inhibitors". Journal of Drugs in Dermatology 11 (5): 609–12. May 2012. PMID 22527429.
- ↑ 78.0 78.1 "Familial aggregation and heritability of sarcoidosis: a Swedish nested case-control study". The European Respiratory Journal 52 (2): 1800385. August 2018. doi:10.1183/13993003.00385-2018. PMID 29946010.
- ↑ "Advances in the genetics of sarcoidosis". Proceedings of the American Thoracic Society 4 (5): 457–60. August 2007. doi:10.1513/pats.200606-136MS. PMID 17684289.
- ↑ "Recent advances in the genetics of sarcoidosis". Journal of Medical Genetics 50 (5): 290–7. May 2013. doi:10.1136/jmedgenet-2013-101532. PMID 23526832.
- ↑ "Human leukocyte antigen class I alleles and the disease course in sarcoidosis patients". American Journal of Respiratory and Critical Care Medicine 169 (6): 696–702. March 2004. doi:10.1164/rccm.200303-459OC. PMID 14656748.
- ↑ "Review: role of genetics in susceptibility and outcome of sarcoidosis". Seminars in Respiratory and Critical Care Medicine 31 (4): 380–9. August 2010. doi:10.1055/s-0030-1262206. PMID 20665388.
- ↑ "Relationship between tumor necrosis factor-α (TNFA) gene polymorphisms and cardiac sarcoidosis". In Vivo 28 (6): 1125–9. 2014-12-01. PMID 25398810.
- ↑ "Etiology of sarcoidosis: does infection play a role?". The Yale Journal of Biology and Medicine 85 (1): 133–41. March 2012. PMID 22461752.
- ↑ 85.0 85.1 85.2 "Pathogenesis of sarcoidosis". Presse Médicale 41 (6 Pt 2): e275–87. June 2012. doi:10.1016/j.lpm.2012.03.018. PMID 22595775.
- ↑ "Molecular evidence for the role of mycobacteria in sarcoidosis: a meta-analysis". The European Respiratory Journal 30 (3): 508–16. September 2007. doi:10.1183/09031936.00002607. PMID 17537780.
- ↑ "Growth of acid fast L forms from the blood of patients with sarcoidosis". Thorax 51 (5): 530–3. May 1996. doi:10.1136/thx.51.5.530. PMID 8711683.
- ↑ "Recent advances in sarcoidosis". Chest 139 (1): 174–82. January 2011. doi:10.1378/chest.10-0188. PMID 21208877. http://journal.publications.chestnet.org/data/Journals/CHEST/20389/100188.pdf. Retrieved 2014-02-20.
- ↑ "Donor-acquired sarcoidosis". Sarcoidosis, Vasculitis, and Diffuse Lung Diseases 19 (1): 18–24. March 2002. PMID 12002380.
- ↑ "Are infectious diseases risk factors for sarcoidosis or a result of reverse causation? Findings from a population-based nested case-control study". European Journal of Epidemiology 35 (11): 1087–1097. November 2020. doi:10.1007/s10654-020-00611-w. PMID 32048110.
- ↑ "The Th1/Th2 paradigm". Immunology Today 18 (6): 263–6. June 1997. doi:10.1016/S0167-5699(97)80019-9. PMID 9190109.
- ↑ "Delayed cutaneous hypersensitivity tests and lymphopenia as activity markers in sarcoidosis". Chest 121 (4): 1239–44. April 2002. doi:10.1378/chest.121.4.1239. PMID 11948059.
- ↑ 93.0 93.1 93.2 "Cytokine modulators in the treatment of sarcoidosis". Rheumatology International 31 (12): 1539–44. December 2011. doi:10.1007/s00296-011-1969-9. PMID 21644041.
- ↑ "The protean face of sarcoidosis revisited". Nephrology, Dialysis, Transplantation 21 (10): 2690–4. October 2006. doi:10.1093/ndt/gfl369. PMID 16861724.
- ↑ "Development of sarcoidosis in etanercept-treated rheumatoid arthritis patients". Clinical Rheumatology 26 (11): 1969–71. November 2007. doi:10.1007/s10067-007-0594-1. PMID 17340045.
- ↑ "Sarcoidosis". New England Journal of Medicine 357 (21): 2153–2165. 2007. doi:10.1056/NEJMra071714. PMID 18032765.
- ↑ van Maarsseveen, T. C. M. Th; Vos, W.; van Diest, P. J. (March 2009). "Giant cell formation in sarcoidosis: cell fusion or proliferation with non-division?". Clinical and Experimental Immunology 155 (3): 476–486. doi:10.1111/j.1365-2249.2008.03841.x. ISSN 1365-2249. PMID 19077083.
- ↑ 98.0 98.1 Mukhopadhyay, Sanjay; Gal, Anthony A. (2010-05-01). "Granulomatous lung disease: an approach to the differential diagnosis". Archives of Pathology & Laboratory Medicine 134 (5): 667–690. doi:10.5858/134.5.667. ISSN 1543-2165. PMID 20441499. https://doi.org/10.5858/134.5.667.
- ↑ Ma, YanLing; Gal, Anthony; Koss, Michael N. (August 2007). "The pathology of pulmonary sarcoidosis: update". Seminars in Diagnostic Pathology 24 (3): 150–161. doi:10.1053/j.semdp.2007.06.002. ISSN 0740-2570. PMID 17882899. https://pubmed.ncbi.nlm.nih.gov/17882899.
- ↑ "Diagnosis of sarcoidosis". Disease-a-Month 55 (11): 693–703. November 2009. doi:10.1016/j.disamonth.2009.06.001. PMID 19857643.
- ↑ "Pulmonary sarcoidosis: the 'Great Pretender'". Clinical Radiology 65 (8): 642–50. August 2010. doi:10.1016/j.crad.2010.03.004. PMID 20599067.
- ↑ "Sarcoidosis--moving to the new standard of diagnosis?". Medicina 46 (7): 443–6. 2010. doi:10.3390/medicina46070063. PMID 20966615. http://medicina.kmu.lt/1007/1007-01e.pdf.
- ↑ 103.0 103.1 103.2 "Sarcoidosis Workup". Medscape Reference. WebMD. 6 February 2014. http://emedicine.medscape.com/article/301914-workup#showall.
- ↑ "Case of the Month". Diagnostic Imaging 31 (9): 10. 2009. http://www.diagnosticimaging.com/display/article/113619/1451377.
- ↑ Joanne Mambretti (2004). "Chest X-ray Stages of Sarcoidosis". Journal of Insurance Medicine: 91–92. http://www.aaimedicine.org/journal-of-insurance-medicine/jim/2004/036-01-0091.pdf. Retrieved June 3, 2012.
- ↑ "Asymptomatic gastrocnemius muscle biopsy: an extremely sensitive and specific test in the pathologic confirmation of sarcoidosis presenting with hilar adenopathy" (PDF). Clinical and Experimental Rheumatology 19 (5): 569–72. 2001. PMID 11579718. http://www.clinexprheumatol.org/article.asp?a=1137.
- ↑ "Diagnosis and treatment of cardiac sarcoidosis". Heart 102 (3): 184–90. February 2016. doi:10.1136/heartjnl-2015-307877. PMID 26643814.
- ↑ "FDG-PET is a Superior Tool in the Diagnosis and Management of Cardiac Sarcoidosis". https://www.acc.org/latest-in-cardiology/articles/2017/04/10/08/43/fdg-pet-is-a-superior-tool.
- ↑ "Treatment of Sarcoidosis". Clinical Reviews in Allergy & Immunology 49 (1): 79–92. August 2015. doi:10.1007/s12016-015-8492-9. PMID 25989728.
- ↑ 110.0 110.1 110.2 110.3 "New treatment strategies for pulmonary sarcoidosis: antimetabolites, biological drugs, and other treatment approaches". The Lancet. Respiratory Medicine 3 (10): 813–22. October 2015. doi:10.1016/S2213-2600(15)00199-X. PMID 26204816.
- ↑ Peters, SP; Talavera, F; Rice, TD et al., eds (6 February 2014). "Sarcoidosis Treatment & Management". Medscape Reference. WebMD. http://emedicine.medscape.com/article/301914-clinical#showall.
- ↑ "Current and emerging strategies for the management of sarcoidosis". Expert Opinion on Pharmacotherapy 8 (9): 1293–311. June 2007. doi:10.1517/14656566.8.9.1293. PMID 17563264.
- ↑ "Corticosteroids for pulmonary sarcoidosis". The Cochrane Database of Systematic Reviews 2010 (2): CD001114. April 2005. doi:10.1002/14651858.CD001114.pub2. PMID 15846612.
- ↑ "Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis". The European Respiratory Journal 38 (5): 1145–50. November 2011. doi:10.1183/09031936.00195010. PMID 21565914.
- ↑ 115.0 115.1 "Acute pulmonary exacerbations of sarcoidosis". Chest 142 (4): 827–836. October 2012. doi:10.1378/chest.12-1060. PMID 23032450. http://journal.publications.chestnet.org/data/Journals/CHEST/25163/chest_142_4_827.pdf. Retrieved 2014-02-20.
- ↑ 116.0 116.1 116.2 "Treatment of sarcoidosis". Disease-a-Month 55 (11): 704–18. November 2009. doi:10.1016/j.disamonth.2009.06.002. PMID 19857644.
- ↑ 117.0 117.1 117.2 117.3 117.4 117.5 117.6 117.7 117.8 "Established and experimental medical therapy of pulmonary sarcoidosis". The European Respiratory Journal 41 (6): 1424–38. June 2013. doi:10.1183/09031936.00060612. PMID 23397302.
- ↑ Rossides, Marios; Kullberg, Susanna; Giuseppe, Daniela Di; Eklund, Anders; Grunewald, Johan; Askling, Johan; Arkema, Elizabeth V. (2021). "Infection risk in sarcoidosis patients treated with methotrexate compared to azathioprine: A retrospective 'target trial' emulated with Swedish real-world data" (in en). Respirology 26 (5): 452–460. doi:10.1111/resp.14001. ISSN 1440-1843. PMID 33398914.
- ↑ "Mycophenolate mofetil therapy for sarcoidosis-associated uveitis". Ocular Immunology and Inflammation 17 (3): 185–90. May–June 2009. doi:10.1080/09273940902862992. PMID 19585361.
- ↑ "Mycophenolate mofetil may be effective in CNS sarcoidosis but not in sarcoid myopathy". Neurology 76 (13): 1168–72. March 2011. doi:10.1212/WNL.0b013e318212aafb. PMID 21444902.
- ↑ "The treatment of pulmonary sarcoidosis". Respiratory Medicine 106 (10): 1351–61. October 2012. doi:10.1016/j.rmed.2012.01.013. PMID 22495110.
- ↑ "Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study". Respiration; International Review of Thoracic Diseases 86 (5): 376–83. 2013. doi:10.1159/000345596. PMID 23295253.
- ↑ "Treatment of refractory neurosarcoidosis with cladribine". The New England Journal of Medicine 350 (17): 1798–9. April 2004. doi:10.1056/NEJMc032345. PMID 15103013.
- ↑ "Efficacy and safety of TNF antagonists in sarcoidosis: data from the Spanish registry of biologics BIOBADASER and a systematic review". Seminars in Arthritis and Rheumatism 42 (1): 89–103. August 2012. doi:10.1016/j.semarthrit.2011.12.006. PMID 22387045.
- ↑ "Targeting the TNF-alpha pathway in sarcoidosis". Expert Opinion on Therapeutic Targets 14 (1): 21–9. January 2010. doi:10.1517/14728220903449244. PMID 20001207.
- ↑ 126.0 126.1 126.2 "Current and emerging pharmacological treatments for sarcoidosis: a review". Drug Design, Development and Therapy 7: 325–38. 2013. doi:10.2147/DDDT.S31064. PMID 23596348.
- ↑ "Tumor necrosis factor-alpha inhibitor treatment for sarcoidosis". Therapeutics and Clinical Risk Management 4 (6): 1305–13. December 2008. doi:10.2147/TCRM.S967. PMID 19337437.
- ↑ 128.0 128.1 128.2 128.3 "Sarcoidosis - a clinically orientated review". Journal of Oral Pathology & Medicine 42 (4): 281–9. April 2013. doi:10.1111/j.1600-0714.2012.01198.x. PMID 22845844.
- ↑ "Sarcoidosis of the liver: to treat or not to treat?" (PDF). The Netherlands Journal of Medicine 70 (8): 349–56. October 2012. PMID 23065982. http://www.njmonline.nl/getpdf.php?id=10000876.
- ↑ "Efficacy and safety of apremilast in chronic cutaneous sarcoidosis". Archives of Dermatology 148 (2): 262–4. February 2012. doi:10.1001/archdermatol.2011.301. PMID 22004880.
- ↑ Clinical trial number NCT01830959 for "Use of Roflumilast to Prevent Exacerbations in Fibrotic Sarcoidosis Patients (REFS)" at ClinicalTrials.gov
- ↑ "Rituximab as a treatment alternative in sarcoidosis". Joint, Bone, Spine 75 (4): 511–2. July 2008. doi:10.1016/j.jbspin.2008.01.025. PMID 18562234.
- ↑ Clinical trial number NCT00279708 for "Atorvastatin to Treat Pulmonary Sarcoidosis" at ClinicalTrials.gov
- ↑ "ACE Inhibitor in the treatment of cutaneous and lymphatic sarcoidosis". American Journal of Clinical Dermatology 8 (3): 183–6. 2007. doi:10.2165/00128071-200708030-00006. PMID 17492847.
- ↑ "Narrative review: fibrotic diseases: cellular and molecular mechanisms and novel therapies". Annals of Internal Medicine 152 (3): 159–66. February 2010. doi:10.7326/0003-4819-152-3-201002020-00007. PMID 20124232.
- ↑ "Nicotine treatment improves Toll-like receptor 2 and Toll-like receptor 9 responsiveness in active pulmonary sarcoidosis" (PDF). Chest 143 (2): 461–470. February 2013. doi:10.1378/chest.12-0383. PMID 22878868. http://journal.publications.chestnet.org/pdfaccess.ashx?ResourceID=5237502&PDFSource=13. Retrieved 2014-02-21.
- ↑ "Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study". JAMA Dermatology 149 (9): 1040–9. September 2013. doi:10.1001/jamadermatol.2013.4646. PMID 23863960.
- ↑ "Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis". Clinical Nutrition 30 (4): 506–12. August 2011. doi:10.1016/j.clnu.2011.01.010. PMID 21324570.
- ↑ 139.0 139.1 139.2 139.3 139.4 139.5 "Consequences of Sarcoidosis". Clinics in Chest Medicine 36 (4): 727–37. December 2015. doi:10.1016/j.ccm.2015.08.013. PMID 26593145.
- ↑ "Does physical training reduce fatigue in sarcoidosis?". Sarcoidosis, Vasculitis, and Diffuse Lung Diseases 32 (1): 53–62. June 2015. PMID 26237356.
- ↑ 141.0 141.1 "Benefits of Physical Training in Sarcoidosis". Lung 193 (5): 701–8. October 2015. doi:10.1007/s00408-015-9784-9. PMID 26286208.
- ↑ "Effects of Inspiratory Muscle Training in Subjects With Sarcoidosis: A Randomized Controlled Clinical Trial". Respiratory Care 61 (4): 483–94. April 2016. doi:10.4187/respcare.04312. PMID 26715771.
- ↑ "Rehabilitation programmes in sarcoidosis: a multidisciplinary approach". Sarcoidosis. 32. 2005. 316–326. doi:10.1183/1025448x.00032021. ISBN 9781904097372.
- ↑ "Sarcoidosis-associated fatigue: an often forgotten symptom--author reply". Expert Review of Clinical Immunology 9 (2): 111. February 2013. doi:10.1586/ECI.12.93. PMID 23390941.
- ↑ "What Is Sarcoidosis?". National Heart, Lung and Blood Institute. National Institutes of Health. 14 June 2013. https://www.nhlbi.nih.gov/health/health-topics/topics/sarc/.
- ↑ 146.0 146.1 146.2 "Sarcoid heart disease". The Canadian Journal of Cardiology 20 (1): 89–93. January 2004. PMID 14968147.
- ↑ "Corticosteroid therapy for cardiac sarcoidosis: a systematic review". The Canadian Journal of Cardiology 29 (9): 1034–41. September 2013. doi:10.1016/j.cjca.2013.02.004. PMID 23623644.
- ↑ 148.0 148.1 "Sarcoidosis mortality in Sweden: a population-based cohort study". The European Respiratory Journal 51 (2): 1701815. February 2018. doi:10.1183/13993003.01815-2017. PMID 29467203.
- ↑ 149.0 149.1 "Risk of first and recurrent serious infection in sarcoidosis: a Swedish register-based cohort study". The European Respiratory Journal 56 (3). September 2020. doi:10.1183/13993003.00767-2020. PMID 32366492.
- ↑ "Long-Term Adverse Cardiac Outcomes in Patients With Sarcoidosis". Journal of the American College of Cardiology 76 (7): 767–777. August 2020. doi:10.1016/j.jacc.2020.06.038. PMID 32792073.
- ↑ "Association between sarcoidosis and lymphoma revisited". Journal of Clinical Pathology 49 (3): 208–12. March 1996. doi:10.1136/jcp.49.3.208. PMID 8675730.
- ↑ "Increased risk for cancer following sarcoidosis". American Journal of Respiratory and Critical Care Medicine 160 (5 Pt 1): 1668–72. November 1999. doi:10.1164/ajrccm.160.5.9904045. PMID 10556138.
- ↑ "Immunopathogenesis of sarcoidosis and risk of malignancy: a lost truth?". International Journal of Immunopathology and Pharmacology 26 (2): 305–13. April–June 2013. doi:10.1177/039463201302600204. PMID 23755746.
- ↑ "Occurrence of sarcoidosis subsequent to chemotherapy for non-Hodgkin's lymphoma: report of two cases". Annals of Hematology 81 (2): 103–5. February 2002. doi:10.1007/s00277-001-0415-6. PMID 11907791.
- ↑ "Two cases of sarcoidosis-lymphoma syndrome". Medical Oncology 24 (4): 469–71. 2007. doi:10.1007/s12032-007-0026-8. PMID 17917102.
- ↑ "Sarcoidosis occurring after lymphoma: report of 14 patients and review of the literature". Medicine 93 (21): e121. November 2014. doi:10.1097/MD.0000000000000121. PMID 25380084.
- ↑ "Benign lesions in cancer patients: Case 1. Sarcoidosis after chemoradiation for head and neck cancer". Journal of Clinical Oncology 23 (3): 640–1. January 2005. doi:10.1200/JCO.2005.02.089. PMID 15659510.
- ↑ "Concurrence of sarcoidosis and lung cancer. A report of four cases". Respiration; International Review of Thoracic Diseases 67 (1): 90–3. 2000. doi:10.1159/000029470. PMID 10705270.
- ↑ "Sarcoidosis as a diagnostic pitfall of pancreatic cancer". Clinical & Translational Oncology 11 (6): 396–8. June 2009. doi:10.1007/s12094-009-0375-1. PMID 19531456.
- ↑ "Hairy cell leukemia and sarcoidosis: a case report and review of the literature". Leukemia 17 (10): 2057–9. October 2003. doi:10.1038/sj.leu.2403074. PMID 14513061.
- ↑ "Acute myeloid leukemia complicating sarcoidosis". Journal of the Royal Society of Medicine 85 (1): 58–9. January 1992. PMID 1548666.
- ↑ "Acute myeloblastic leukemia and sarcoidosis. Implications for pathogenesis". Cancer 55 (2): 366–9. January 1985. doi:10.1002/1097-0142(19850115)55:2<366::AID-CNCR2820550212>3.0.CO;2-1. PMID 3855267.
- ↑ "The spectrum of opportunistic diseases complicating sarcoidosis". Autoimmunity Reviews 14 (1): 64–74. January 2015. doi:10.1016/j.autrev.2014.10.006. PMID 25305373.
- ↑ "Progressive multifocal leukoencephalopathy in patients with sarcoidosis". Neurology 82 (15): 1307–13. April 2014. doi:10.1212/WNL.0000000000000318. PMID 24610328.
- ↑ Rapid Medicine. Wiley-Blackwell. 2010. ISBN 978-1405183239.
- ↑ "The epidemiology of sarcoidosis in Rochester, Minnesota: a population-based study of incidence and survival". American Journal of Epidemiology 123 (5): 840–5. May 1986. doi:10.1093/oxfordjournals.aje.a114313. PMID 3962966.
- ↑ "Prevalence of coeliac disease in patients with sarcoidosis". European Journal of Gastroenterology & Hepatology 16 (9): 911–5. September 2004. doi:10.1097/00042737-200409000-00016. PMID 15316417.
- ↑ 168.0 168.1 "Sarcoidosis". Lancet 361 (9363): 1111–8. March 2003. doi:10.1016/S0140-6736(03)12888-7. PMID 12672326.
- ↑ 169.0 169.1 "Sarcoidosis: epidemiology, etiology, pathogenesis, and genetics". Disease-a-Month 55 (11): 649–60. November 2009. doi:10.1016/j.disamonth.2009.04.008. PMID 19857640.
- ↑ 170.0 170.1 170.2 170.3 170.4 170.5 170.6 "Chapter 1: Definition and history of sarcoidosis". Sarcoidosis.. Sheffield: European Respiratory Society Journals. 2005. ISBN 9781904097884.
- ↑ "Disease of Besnier-Boeck-Schaumann". New England Journal of Medicine 220 (4): 143–145. 26 January 1939. doi:10.1056/NEJM193901262200404.
- ↑ "Join WASOG". wasog.org. http://www.wasog.org/.
- ↑ "Index". Sarcoidosis, Vasculitis and Diffuse Lung Diseases. 2016. http://www.mattioli1885journals.com/index.php/sarcoidosis/index. Retrieved 9 April 2016.
- ↑ "Mission & Goals". Foundation for Sarcoidosis Research. http://www.stopsarcoidosis.org/about-us/mission_goals/.
- ↑ "World Trade Center "sarcoid-like" granulomatous pulmonary disease in New York City Fire Department rescue workers". Chest 131 (5): 1414–23. May 2007. doi:10.1378/chest.06-2114. PMID 17400664. http://journal.publications.chestnet.org/data/Journals/CHEST/22056/1414.pdf. Retrieved 2014-02-22.
- ↑ "9/11 Health – What We Know About the Health Effects of 9/11". NYC. US Government. http://www.nyc.gov/html/doh/wtc/html/know/physical.shtml.
- ↑ "Bernie Mac, Acerbic Stand-Up Comedian and Irascible TV Dad, Dies at 50". The New York Times. 10 August 2008. https://www.nytimes.com/2008/08/10/arts/television/10mac.html.
- ↑ "Actor and comedian Bernie Mac dies at age 58". CBS2Chicago. August 9, 2008. http://cbs2chicago.com/local/bernie.mac.dead.2.791473.html.
- ↑ Kat Carney (September 19, 2003). Former MTV VJ tells of battle with chronic illness CNN.com, accessed 10 August 2019
- ↑ "Thursday roundup: Maddox rides to Ben's defense". May 20, 2005. http://www.espn.com/nfl/news/story?id=2063708.
- ↑ Donna J. Miller (????) Coroner says singer Sean Levert died of natural causes. Cleveland.com, accessed 11 August 2019
- ↑ Zolan Kanno-Youngs (12 September 2017). "Wall Street Journal's Joseph Rago Died of Natural Causes, Medical Examiner Says". The Wall Street Journal. https://www.wsj.com/articles/medical-examiner-said-wall-street-journals-joseph-rago-died-of-natural-causes-1505242044.
- ↑ "Robespierre: the oldest case of sarcoidosis?". Lancet 382 (9910): 2068. December 2013. doi:10.1016/S0140-6736(13)62694-X. PMID 24360387.
- ↑ Mai FM (2006). "Beethoven's terminal illness and death". The Journal of the Royal College of Physicians of Edinburgh 36 (3): 258–63. PMID 17214130.
- ↑ Sharma OP (2005). "Murray Kornfeld, American College of Chest Physician, and sarcoidosis: a historical footnote: 2004 Murray Kornfeld Memorial Founders Lecture". Chest 128 (3): 1830–35. doi:10.1378/chest.128.3.1830. PMID 16162793.
- ↑ "Pregnancy and sarcoidosis: an insight into the pathogenesis of hypercalciuria". Chest 126 (3): 995–8. September 2004. doi:10.1378/chest.126.3.995. PMID 15364785.
- ↑ 187.0 187.1 "Maternal and infant outcomes in sarcoidosis pregnancy: a Swedish population-based cohort study of first births". Respiratory Research 21 (1): 225. August 2020. doi:10.1186/s12931-020-01493-y. PMID 32854707.
External links
| Classification | |
|---|---|
| External resources |
- Sarcoidosis UK Information Hub
- Iannuzzi, M. C.; Sah, B. P. (March 2008). Sarcoidosis: Interstitial Lung Diseases. Merck Sharp & Dohme Corp. http://www.merckmanuals.com/home/lung_and_airway_disorders/interstitial_lung_diseases/sarcoidosis.html. Retrieved 19 February 2014.
 |