Medicine:Coronavirus disease 2019
| Coronavirus disease 2019 (COVID-19) | |
|---|---|
| Other names | |
 | |
False color transmission electron microscope image of Coronavirus | |
| Pronunciation | |
| Specialty | Infectious disease |
| Symptoms | Fever, cough, fatigue, shortness of breath, loss of smell; sometimes no symptoms at all[5][6] |
| Complications | Pneumonia, viral sepsis, acute respiratory distress syndrome, kidney failure, cytokine release syndrome |
| Usual onset | 2–14 days (typically 5) from infection |
| Causes | Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) |
| Diagnostic method | rRT-PCR testing, CT scan |
| Prevention | Hand washing, face coverings, quarantine, social distancing[7] |
| Treatment | Symptomatic and supportive |
| Frequency | 285,043,163[8] confirmed cases |
| Deaths | 5,425,817 (1.90% of confirmed cases)[8] |
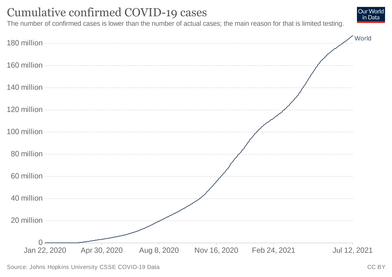
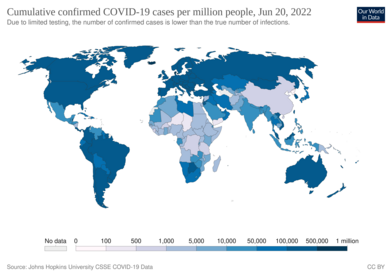
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).[9] It was first identified in December 2019 in Wuhan, Hubei, China, and has resulted in an ongoing pandemic.[10][11] The first confirmed case has been traced back to 17 November 2019 in Hubei.[12] As of 30 December 2021, more than 285 million cases have been reported across countries and territories, resulting in more than 5.42 million deaths. More than people have recovered.[8]
Common symptoms include fever, cough, fatigue, shortness of breath, and loss of smell and taste.[13][5][6][14] While the majority of cases result in mild symptoms, some progress to acute respiratory distress syndrome (ARDS) possibly precipitated by cytokine storm,[15] multi-organ failure, septic shock, and blood clots.[16][17][18] The time from exposure to onset of symptoms is typically around five days, but may range from two to fourteen days.[5][19]
<section begin="Spread"/>The virus is primarily spread between people during close contact,[lower-alpha 1] most often via small droplets produced by coughing,[lower-alpha 2] sneezing, and talking.[6][20][22] The droplets usually fall to the ground or onto surfaces rather than travelling through air over long distances.[6][23] Transmission may also occur through smaller droplets that are able to stay suspended in the air for longer periods of time.[24] Less commonly, people may become infected by touching a contaminated surface and then touching their face.[6][20] It is most contagious during the first three days after the onset of symptoms, although spread is possible before symptoms appear, and from people who do not show symptoms.[6][20]<section end="Spread" /> The standard method of diagnosis is by real-time reverse transcription polymerase chain reaction (rRT-PCR) from a nasopharyngeal swab.[25] Chest CT imaging may also be helpful for diagnosis in individuals where there is a high suspicion of infection based on symptoms and risk factors; however, guidelines do not recommend using CT imaging for routine screening.[26][27]
Recommended measures to prevent infection include frequent hand washing, maintaining physical distance from others (especially from those with symptoms), quarantine (especially for those with symptoms), covering coughs, and keeping unwashed hands away from the face.[7][28][29] The use of cloth face coverings such as a scarf or a bandana has been recommended by health officials in public settings to minimise the risk of transmissions, with some authorities requiring their use.[30][31] Health officials also stated that medical-grade face masks, such as N95 masks, should only be used by healthcare workers, first responders, and those who directly care for infected individuals.[32][33]
There are no vaccines nor specific antiviral treatments for COVID-19.[6] Management involves the treatment of symptoms, supportive care, isolation, and experimental measures.[34] The World Health Organization (WHO) declared the COVID‑19 outbreak a public health emergency of international concern (PHEIC)[35][36] on 30 January 2020 and a pandemic on 11 March 2020.[11] Local transmission of the disease has occurred in most countries across all six WHO regions.[37] thumb|thumbtime=0:02|upright=1.4|Video summary (script)
Signs and symptoms
| Symptom | Range |
|---|---|
| Fever | 83–99% |
| Cough | 59–82% |
| Loss of appetite | 40–84% |
| Fatigue | 44–70% |
| Shortness of breath | 31–40% |
| Coughing up sputum | 28–33% |
| Muscle aches and pains | 11–35% |
Fever is the most common symptom of COVID-19,[13] but is highly variable in severity and presentation, with some older, immunocompromised, or critically ill people not having fever at all.[39][40] In one study, only 44% of people had fever when they presented to the hospital, while 89% went on to develop fever at some point during their hospitalization.[41]
Other common symptoms include cough, loss of appetite, fatigue, shortness of breath, sputum production, and muscle and joint pains.[13][1][5][42] Symptoms such as nausea, vomiting, and diarrhoea have been observed in varying percentages.[43][44][45] Less common symptoms include sneezing, runny nose, sore throat, and skin lesions.[46] Some cases in China initially presented with only chest tightness and palpitations.[47] A decreased sense of smell or disturbances in taste may occur.[48][49] Loss of smell was a presenting symptom in 30% of confirmed cases in South Korea.[14][50]
As is common with infections, there is a delay between the moment a person is first infected and the time he or she develops symptoms. This is called the incubation period. The typical incubation period for COVID‑19 is five or six days, but it can range from one to fourteen days[6][51] with approximately ten percent of cases taking longer.[52][53][54]
An early key to the diagnosis is the tempo of the illness. Early symptoms may include a wide variety of symptoms but infrequently involves shortness of breath. Shortness of breath usually develops several days after initial symptoms. Shortness of breath that begins immediately along with fever and cough is more likely to be anxiety than COVID-19. The most critical days of illness tend to be those following the development of shortness of breath.[55] A minority of cases do not develop noticeable symptoms at any point in time.[56] These asymptomatic carriers tend not to get tested, and their role in transmission is not fully known.[57][58] Preliminary evidence suggested they may contribute to the spread of the disease.[59] In June 2020, a spokeswoman of WHO said that asymptomatic transmission appears to be "rare," but the evidence for the claim was not released.[60] The next day, WHO clarified that they had intended a narrow definition of "asymptomatic" that did not include pre-symptomatic or paucisymptomatic (weak symptoms) transmission and that up to 41% of transmission may be asymptomatic. Transmission without symptoms does occur.[56]
Cause
Transmission
<section begin="Transmission"/> alt=Cough/sneeze droplets visualised in dark background using Tyndall scattering|thumb|Respiratory droplets produced when a man sneezes, visualised using Tyndall scattering
COVID‑19 is a new disease, and many of the details of its spread are still under investigation.[6][20][22] It spreads easily between people—easier than influenza but not as easily as measles.[20] People are most infectious when they show symptoms (even mild or non-specific symptoms), but may be infectious for up to two days before symptoms appear (pre-symptomatic transmission).[22] They remain infectious an estimated seven to twelve days in moderate cases and an average of two weeks in severe cases.[22] People can also transmit the virus without showing any symptom (asymptomatic transmission), but it is unclear how often this happens.[6][20][22] A June 2020 review found that 40–45% of infected people are asymptomatic.[61]
COVID-19 spreads primarily when people are in close contact and one person inhales small droplets produced by an infected person (symptomatic or not) coughing, sneezing, talking, or singing.[22][62] The WHO recommends 1 metre (3 ft) of social distance;[6] the US Centers for Disease Control and Prevention (CDC) recommends 2 metres (6 ft).[20]
Transmission may also occur through aerosols, smaller droplets that are able to stay suspended in the air for longer periods of time.[24] Experimental results show the virus can survive in aerosol up to three hours.[63] Some outbreaks have also been reported in crowded and inadequately ventilated indoor locations where infected persons spend long periods of time (such as restaurants and nightclubs).[64] Aerosol transmission in such locations has not been ruled out.[24] Some medical procedures performed on COVID-19 patients in health facilities can generate those smaller droplets,[65] and result in the virus being transmitted more easily than normal.[6][22]
When the contaminated droplets fall to floors or surfaces they can, though less commonly, remain infectious if people touch contaminated surfaces and then their eyes, nose or mouth with unwashed hands.[6] On surfaces the amount of viable active virus decreases over time until it can no longer cause infection,[22] and surfaces are thought not to be the main way the virus spreads.[20] It is unknown what amount of virus on surfaces is required to cause infection via this method, but it can be detected for up to four hours on copper, up to one day on cardboard, and up to three days on plastic (polypropylene) and stainless steel (AISI 304).[22][66][67] Surfaces are easily decontaminated with household disinfectants which destroy the virus outside the human body or on the hands.[6] Disinfectants or bleach are not a treatment for COVID‑19, and cause health problems when not used properly, such as when used inside the human body.[68]
Sputum and saliva carry large amounts of virus.[6][20][22][69] Although COVID‑19 is not a sexually transmitted infection, direct contact such as kissing, intimate contact, and fecal–oral routes are suspected to transmit the virus.[70][71] The virus may occur in breast milk, but it's unknown whether it's infectious and transmittable to the baby.[72][73]
Estimates of the number of people infected by one person with COVID-19, the R0, have varied. The WHO's initial estimates of R0 were 1.4–2.5 (average 1.95), however an early April 2020 review found the basic R0 (without control measures) to be higher at 3.28 and the median R0 to be 2.79.[74]<section end="Transmission" />
Virology
thumb|Illustration of SARSr-CoV virion
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel severe acute respiratory syndrome coronavirus, first isolated from three people with pneumonia connected to the cluster of acute respiratory illness cases in Wuhan.[75] All features of the novel SARS-CoV-2 virus occur in related coronaviruses in nature.[76] Outside the human body, the virus is destroyed by household soap, which bursts its protective bubble.[26]
SARS-CoV-2 is closely related to the original SARS-CoV.[77] It is thought to have an animal (zoonotic) origin. Genetic analysis has revealed that the coronavirus genetically clusters with the genus Betacoronavirus, in subgenus Sarbecovirus (lineage B) together with two bat-derived strains. It is 96% identical at the whole genome level to other bat coronavirus samples (BatCov RaTG13).[46] In February 2020, Chinese researchers found that there is only one amino acid difference in the binding domain of the S protein between the coronaviruses from pangolins and those from humans; however, whole-genome comparison to date[when?] found that at most 92% of genetic material was shared between pangolin coronavirus and SARS-CoV-2, which is insufficient to prove pangolins to be the intermediate host.[78]
Pathophysiology
The lungs are the organs most affected by COVID‑19 because the virus accesses host cells via the enzyme angiotensin-converting enzyme 2 (ACE2), which is most abundant in type II alveolar cells of the lungs.[79] The virus uses a special surface glycoprotein called a "spike" (peplomer) to connect to ACE2 and enter the host cell.[80] The density of ACE2 in each tissue correlates with the severity of the disease in that tissue and some have suggested decreasing ACE2 activity might be protective,[81][82][unreliable medical source?] though another view is that increasing ACE2 using angiotensin II receptor blocker medications could be protective.[83] As the alveolar disease progresses, respiratory failure might develop and death may follow.[82][unreliable medical source?]
SARS-CoV-2 may also cause respiratory failure through affecting the brainstem as other coronaviruses have been found to invade the central nervous system (CNS). While virus has been detected in cerebrospinal fluid of autopsies, the exact mechanism by which it invades the CNS remains unclear and may first involve invasion of peripheral nerves given the low levels of ACE2 in the brain.[84][85][unreliable medical source?]
The virus also affects gastrointestinal organs as ACE2 is abundantly expressed in the glandular cells of gastric, duodenal and rectal epithelium[86] as well as endothelial cells and enterocytes of the small intestine.[87][unreliable medical source?]
The virus can cause acute myocardial injury and chronic damage to the cardiovascular system.[88] An acute cardiac injury was found in 12% of infected people admitted to the hospital in Wuhan, China,[44] and is more frequent in severe disease.[89][unreliable medical source?] Rates of cardiovascular symptoms are high, owing to the systemic inflammatory response and immune system disorders during disease progression, but acute myocardial injuries may also be related to ACE2 receptors in the heart.[88] ACE2 receptors are highly expressed in the heart and are involved in heart function.[88][90] A high incidence of thrombosis (31%) and venous thromboembolism (25%) have been found in ICU patients with COVID‑19 infections, and may be related to poor prognosis.[91][unreliable medical source?][92][unreliable medical source?] Blood vessel dysfunction and clot formation (as suggested by high D-dimer levels) are thought to play a significant role in mortality, incidences of clots leading to pulmonary embolisms, and ischaemic events within the brain have been noted as complications leading to death in patients infected with SARS-CoV-2. Infection appears to set off a chain of vasoconstrictive responses within the body, constriction of blood vessels within the pulmonary circulation has also been posited as a mechanism in which oxygenation decreases alongside the presentation of viral pneumonia.[93][better source needed]
Another common cause of death is complications related to the kidneys.[93][better source needed] Early reports show that up to 30% of hospitalized patients in both China and New York have experienced some injury to their kidneys, including some persons with no previous kidney problems.[94]
Autopsies of people who died of COVID‑19 have found diffuse alveolar damage (DAD), and lymphocyte-containing inflammatory infiltrates within the lung.[95][unreliable medical source?]
Immunopathology
Although SARS-CoV-2 has a tropism for ACE2-expressing epithelial cells of the respiratory tract, patients with severe COVID‑19 have symptoms of systemic hyperinflammation. Clinical laboratory findings of elevated IL-2, IL-7, IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ inducible protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1-α (MIP-1α), and tumour necrosis factor-α (TNF-α) indicative of cytokine release syndrome (CRS) suggest an underlying immunopathology.[44]
Additionally, people with COVID‑19 and acute respiratory distress syndrome (ARDS) have classical serum biomarkers of CRS, including elevated C-reactive protein (CRP), lactate dehydrogenase (LDH), D-dimer, and ferritin.[96]
Systemic inflammation results in vasodilation, allowing inflammatory lymphocytic and monocytic infiltration of the lung and the heart. In particular, pathogenic GM-CSF-secreting T-cells were shown to correlate with the recruitment of inflammatory IL-6-secreting monocytes and severe lung pathology in COVID‑19 patients.[citation needed] Lymphocytic infiltrates have also been reported at autopsy.[95][unreliable medical source?]
Diagnosis

thumb|US CDC rRT-PCR test kit for COVID-19[97]
The WHO has published several testing protocols for the disease.[98] The standard method of testing is real-time reverse transcription polymerase chain reaction (rRT-PCR).[99] The test is typically done on respiratory samples obtained by a nasopharyngeal swab; however, a nasal swab or sputum sample may also be used.[25][100] Results are generally available within a few hours to two days.[101][102] Blood tests can be used, but these require two blood samples taken two weeks apart, and the results have little immediate value.[103] Chinese scientists were able to isolate a strain of the coronavirus and publish the genetic sequence so laboratories across the world could independently develop polymerase chain reaction (PCR) tests to detect infection by the virus.[10][104][105] (As of April 2020), antibody tests (which may detect active infections and whether a person had been infected in the past) were in development, but not yet widely used.[106][107][108] Antibody tests may be most accurate 2–3 weeks after a person's symptoms start.[109] The Chinese experience with testing has shown the accuracy is only 60 to 70%.[110] The US Food and Drug Administration (FDA) approved the first point-of-care test on 21 March 2020 for use at the end of that month.[111] The absence or presence of COVID-19 signs and symptoms alone is not reliable enough for an accurate diagnosis.[112]
Diagnostic guidelines released by Zhongnan Hospital of Wuhan University suggested methods for detecting infections based upon clinical features and epidemiological risk. These involved identifying people who had at least two of the following symptoms in addition to a history of travel to Wuhan or contact with other infected people: fever, imaging features of pneumonia, normal or reduced white blood cell count, or reduced lymphocyte count.[113]
A study asked hospitalised COVID‑19 patients to cough into a sterile container, thus producing a saliva sample, and detected the virus in eleven of twelve patients using RT-PCR. This technique has the potential of being quicker than a swab and involving less risk to health care workers (collection at home or in the car).[69]
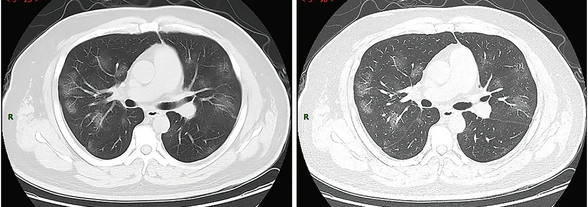
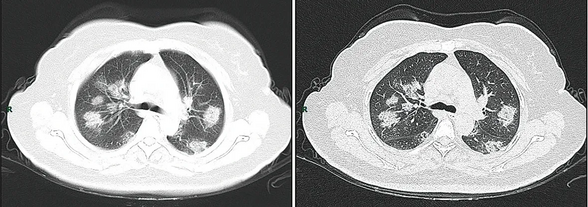
Along with laboratory testing, chest CT scans may be helpful to diagnose COVID‑19 in individuals with a high clinical suspicion of infection but are not recommended for routine screening.[26][27] Bilateral multilobar ground-glass opacities with a peripheral, asymmetric, and posterior distribution are common in early infection.[26] Subpleural dominance, crazy paving (lobular septal thickening with variable alveolar filling), and consolidation may appear as the disease progresses.[26][114]
In late 2019, the WHO assigned emergency ICD-10 disease codes U07.1 for deaths from lab-confirmed SARS-CoV-2 infection and U07.2 for deaths from clinically or epidemiologically diagnosed COVID‑19 without lab-confirmed SARS-CoV-2 infection.[115]
Pathology
Few data are available about microscopic lesions and the pathophysiology of COVID‑19.[116][117] The main pathological findings at autopsy are:[citation needed]
- Macroscopy: pleurisy, pericarditis, lung consolidation and pulmonary oedema
- Four types of severity of viral pneumonia can be observed:
- minor pneumonia: minor serous exudation, minor fibrin exudation
- mild pneumonia: pulmonary oedema, pneumocyte hyperplasia, large atypical pneumocytes, interstitial inflammation with lymphocytic infiltration and multinucleated giant cell formation
- severe pneumonia: diffuse alveolar damage (DAD) with diffuse alveolar exudates. DAD is the cause of acute respiratory distress syndrome (ARDS) and severe hypoxemia.
- healing pneumonia: organisation of exudates in alveolar cavities and pulmonary interstitial fibrosis
- plasmocytosis in BAL[118]
- Blood: disseminated intravascular coagulation (DIC);[119] leukoerythroblastic reaction[120]
- Liver: microvesicular steatosis[citation needed]
Prevention
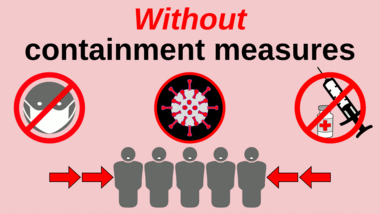
thumb|upright=1.5|Progressively stronger mitigation efforts to reduce the number of active cases at any given time—"[[flattening the curve"—allows healthcare services to better manage the same volume of patients.[122][123][124] Likewise, progressively greater increases in healthcare capacity—called raising the line—such as by increasing bed count, personnel, and equipment, helps to meet increased demand.[125]]] thumb|upright=1.5|Mitigation attempts that are inadequate in strictness or duration—such as premature relaxation of distancing rules or stay-at-home orders—can allow a resurgence after the initial surge and mitigation.[123][126]
A COVID-19 vaccine is not expected until 2021 at the earliest.[127] The US National Institutes of Health guidelines do not recommend any medication for prevention of COVID‑19, before or after exposure to the SARS-CoV-2 virus, outside the setting of a clinical trial.[128][129] Without a vaccine, other prophylactic measures, or effective treatments, a key part of managing COVID‑19 is trying to decrease and delay the epidemic peak, known as "flattening the curve".[123] This is done by slowing the infection rate to decrease the risk of health services being overwhelmed, allowing for better treatment of current cases, and delaying additional cases until effective treatments or a vaccine become available.[123][126]
Preventive measures to reduce the chances of infection include staying at home, wearing a mask in public, avoiding crowded places, keeping distance from others, washing hands with soap and water often and for at least 20 seconds, practising good respiratory hygiene, and avoiding touching the eyes, nose, or mouth with unwashed hands.[130][131][132][133]
The US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) recommend individuals wear non-medical face coverings in public settings where there is an increased risk of transmission and where social distancing measures are difficult to maintain.[134][30][135] This recommendation is meant to reduce the spread of the disease by asymptomatic and pre-symtomatic individuals and is complementary to established preventive measures such as social distancing.[30][136] Face coverings limit the volume and travel distance of expiratory droplets dispersed when talking, breathing, and coughing.[30][136] Many countries and local jurisdictions encourage or mandate the use of face masks or cloth face coverings by members of the public to limit the spread of the virus.[137][138][139][140]
Masks are also strongly recommended for those who may have been infected and those taking care of someone who may have the disease.[141] When not wearing a mask, the CDC recommends covering the mouth and nose with a tissue when coughing or sneezing and recommends using the inside of the elbow if no tissue is available.[131] Proper hand hygiene after any cough or sneeze is encouraged.[131]
Social distancing strategies aim to reduce contact of infected persons with large groups by closing schools and workplaces, restricting travel, and cancelling large public gatherings.[142] Distancing guidelines also include that people stay at least 6 feet (1.8 m) apart.[143] After the implementation of social distancing and stay-at-home orders, many regions have been able to sustain an effective transmission rate ("Rt") of less than one, meaning the disease is in remission in those areas.[144]
The CDC also recommends that individuals wash hands often with soap and water for at least 20 seconds, especially after going to the toilet or when hands are visibly dirty, before eating and after blowing one's nose, coughing or sneezing. The CDC further recommends using an alcohol-based hand sanitiser with at least 60% alcohol, but only when soap and water are not readily available.[131] For areas where commercial hand sanitisers are not readily available, the WHO provides two formulations for local production. In these formulations, the antimicrobial activity arises from ethanol or isopropanol. Hydrogen peroxide is used to help eliminate bacterial spores in the alcohol; it is "not an active substance for hand antisepsis". Glycerol is added as a humectant.[145]
Those diagnosed with COVID‑19 or who believe they may be infected are advised by the CDC to stay home except to get medical care, call ahead before visiting a healthcare provider, wear a face mask before entering the healthcare provider's office and when in any room or vehicle with another person, cover coughs and sneezes with a tissue, regularly wash hands with soap and water and avoid sharing personal household items.[32][146]
Sanitizing of frequently touched surfaces is also recommended or required by regulation for businesses and public facilities; the United States Environmental Protection Agency maintains a list of products expected to be effective.[147]
On 7 July 2020, the WHO said in a press conference that it will issue new guidelines about airborne transmission in settings with close contact and poor ventilation.[148]
For health care professionals who may come into contact with COVID-19 positive bodily fluids, using personal protective coverings on exposed body parts improves protection from the virus.[149] Breathable personal protective equipment improves user-satisfaction and may offer a similar level of protection from the virus.[149] In addition, adding tabs and other modifications to the protective equipment may reduce the risk of contamination during donning and doffing (putting on and taking off the equipment).[149] Implementing an evidence-based donning and doffing protocol such as a one-step glove and gown removal technique, giving oral instructions while donning and doffing, double gloving, and the use of glove disinfection may also improve protection for health care professionals.[149]
Management
People are managed with supportive care, which may include fluid therapy, oxygen support, and supporting other affected vital organs.[150][151][152] The CDC recommends those who suspect they carry the virus wear a simple face mask.[32] Extracorporeal membrane oxygenation (ECMO) has been used to address the issue of respiratory failure, but its benefits are still under consideration.[citation needed][153] Personal hygiene and a healthy lifestyle and diet have been recommended to improve immunity.[154] Supportive treatments may be useful in those with mild symptoms at the early stage of infection.[155]
The WHO, the Chinese National Health Commission, and the United States' National Institutes of Health have published recommendations for taking care of people who are hospitalised with COVID‑19.[128][156][157] Intensivists and pulmonologists in the US have compiled treatment recommendations from various agencies into a free resource, the IBCC.[158][159]
Prognosis
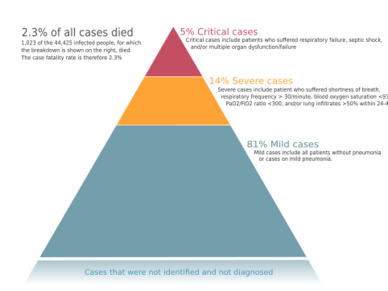
The severity of COVID‑19 varies. The disease may take a mild course with few or no symptoms, resembling other common upper respiratory diseases such as the common cold. Mild cases typically recover within two weeks, while those with severe or critical diseases may take three to six weeks to recover. Among those who have died, the time from symptom onset to death has ranged from two to eight weeks.[46]
Children make up a small proportion of reported cases, with about 1% of cases being under 10 years and 4% aged 10–19 years.[22] They are likely to have milder symptoms and a lower chance of severe disease than adults. In those younger than 50 years the risk of death is less than 0.5%, while in those older than 70 it is more than 8%.[166][167][168] Pregnant women may be at higher risk of severe COVID‑19 infection based on data from other similar viruses, like severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), but data for COVID‑19 is lacking.[169][170] According to scientific reviews smokers are more likely to require intensive care or die compared to non-smokers,[171][172] air pollution is similarly associated with risk factors,[172] and obesity contributes to an increased health risk of COVID-19.[172][173][174]
A European multinational study of hospitalized children published in The Lancet on June 25, 2020 found that about 8% of children admitted to a hospital needed intensive care. 4 out of 582 children (0.7%) died, although the actual mortality rate could be “substantially lower” since milder cases that did not seek medical help were not included in the study.[175]
Comorbidities
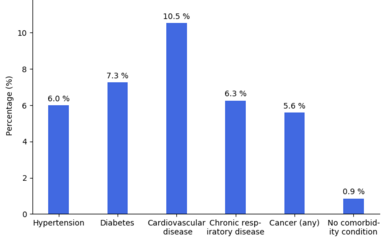
Most of those who die of COVID‑19 have pre-existing (underlying) conditions, including hypertension, diabetes mellitus, and cardiovascular disease.[176] The Istituto Superiore di Sanità reported that out of 8.8% of deaths where medical charts were available, 97% of people had at least one comorbidity with the average person having 2.7 diseases.[177] According to the same report, the median time between the onset of symptoms and death was ten days, with five being spent hospitalised. However, people transferred to an ICU had a median time of seven days between hospitalisation and death.[177] In a study of early cases, the median time from exhibiting initial symptoms to death was 14 days, with a full range of six to 41 days.[178] In a study by the National Health Commission (NHC) of China, men had a death rate of 2.8% while women had a death rate of 1.7%.[179] In 11.8% of the deaths reported by the National Health Commission of China, heart damage was noted by elevated levels of troponin or cardiac arrest.[47] According to March data from the United States, 89% of those hospitalised had preexisting conditions.[180]
Most critical respiratory comorbidities according to the CDC, are: moderate or severe Asthma, pre-existing COPD, pulmonary fibrosis, cystic fibrosis.[181] Current evidence stemming from meta - analysis of several smaller research papers, also suggest that smoking can be assosiated with worse patient outcomes [182][183] When someone with existing respiratory problems is infected with COVID-19, they might be at greater risk for severe symptoms.[184] COVID-19 also poses a greater risk to people who misuse opioids and methamphetamines, insofar as their drug use may have caused lung damage.[185]
Complications and long-term effects
Complications may include pneumonia, acute respiratory distress syndrome (ARDS), multi-organ failure, septic shock, and death.[10][16][186][187] Cardiovascular complications may include heart failure, arrhythmias, heart inflammation, and blood clots.[188]
Approximately 20–30% of people who present with COVID‑19 have elevated liver enzymes reflecting liver injury.[189][129]
Neurologic manifestations include seizure, stroke, encephalitis, and Guillain–Barré syndrome (which includes loss of motor functions).[190] Following the infection, children may develop paediatric multisystem inflammatory syndrome, which has symptoms similar to Kawasaki disease, which can be fatal.[191][192]
Concerns have been raised about long-term sequelae of the disease. The Hong Kong Hospital Authority found a drop of 20% to 30% in lung capacity in some people who recovered from the disease, and lung scans suggested organ damage.[193] This may also lead to post-intensive care syndrome following recovery.[194]
Immunity
It is unknown (as of April 2020) if past infection provides effective and long-term immunity in people who recover from the disease.[195][196] Some of the infected have been reported to develop protective antibodies, so acquired immunity is presumed likely, based on the behaviour of other coronaviruses.[197] Cases in which recovery from COVID‑19 was followed by positive tests for coronavirus at a later date have been reported.[198][199][200][201] However, these cases are believed to be lingering infection rather than reinfection,[201] or false positives due to remaining RNA fragments.[202] An investigation by the Korean CDC of 285 individuals who tested positive for SARS-CoV-2 in PCR tests administered days or weeks after recovery from COVID-19 found no evidence that these individuals were contagious at this later time.[203] Some other coronaviruses circulating in people are capable of reinfection after roughly a year.[204][205]
History
The virus is thought to be natural and has an animal origin,[76] through spillover infection.[206] The first known human infections were in China. A study of the first 41 cases of confirmed COVID‑19, published in January 2020 in The Lancet, reported the earliest date of onset of symptoms as 1 December 2019.[207][208][209] Official publications from the WHO reported the earliest onset of symptoms as 8 December 2019.[210] Human-to-human transmission was confirmed by the WHO and Chinese authorities by 20 January 2020.[211][212] According to official Chinese sources, these were mostly linked to the Huanan Seafood Wholesale Market, which also sold live animals.[213] In May 2020, George Gao, the director of the Chinese Center for Disease Control and Prevention, said animal samples collected from the seafood market had tested negative for the virus, indicating that the market was the site of an early superspreading event, but it was not the site of the initial outbreak.[214] Traces of the virus have been found in wastewater that was collected from Milan and Turin, Italy, on 18 December 2019.[215]
There are several theories about where the very first case (the so-called patient zero) originated.[216] According to an unpublicised report from the Chinese government, the first case can be traced back to 17 November 2019; the person was a 55-year old citizen in the Hubei province. There were four men and five women reported to be infected in November, but none of them were "patient zero".[12] By December 2019, the spread of infection was almost entirely driven by human-to-human transmission.[161][217] The number of coronavirus cases in Hubei gradually increased, reaching 60 by 20 December[218] and at least 266 by 31 December.[219] On 24 December, Wuhan Central Hospital sent a bronchoalveolar lavage fluid (BAL) sample from an unresolved clinical case to sequencing company Vision Medicals. On 27 and 28 December, Vision Medicals informed the Wuhan Central Hospital and the Chinese CDC of the results of the test, showing a new coronavirus.[220] A pneumonia cluster of unknown cause was observed on 26 December and treated by the doctor Zhang Jixian in Hubei Provincial Hospital, who informed the Wuhan Jianghan CDC on 27 December.[221] On 30 December, a test report addressed to Wuhan Central Hospital, from company CapitalBio Medlab, stated an erroneous positive result for SARS, causing a group of doctors at Wuhan Central Hospital to alert their colleagues and relevant hospital authorities of the result. That evening, the Wuhan Municipal Health Commission issued a notice to various medical institutions on "the treatment of pneumonia of unknown cause".[222] Eight of these doctors, including Li Wenliang (punished on 3 January),[223] were later admonished by the police for spreading false rumours, and another, Ai Fen, was reprimanded by her superiors for raising the alarm.[224]
The Wuhan Municipal Health Commission made the first public announcement of a pneumonia outbreak of unknown cause on 31 December, confirming 27 cases[225][226][227]—enough to trigger an investigation.[228]
During the early stages of the outbreak, the number of cases doubled approximately every seven and a half days.[229] In early and mid-January 2020, the virus spread to other Chinese provinces, helped by the Chinese New Year migration and Wuhan being a transport hub and major rail interchange.[230] On 20 January, China reported nearly 140 new cases in one day, including two people in Beijing and one in Shenzhen.[231] Later official data shows 6,174 people had already developed symptoms by then,[232] and more may have been infected.[233] A report in The Lancet on 24 January indicated human transmission, strongly recommended personal protective equipment for health workers, and said testing for the virus was essential due to its "pandemic potential".[234][235] On 30 January, the WHO declared the coronavirus a public health emergency of international concern.[233] By this time, the outbreak spread by a factor of 100 to 200 times.[236]
On 31 January 2020, Italy had its first confirmed cases, two tourists from China.[237] As of 13 March 2020, the WHO considered Europe the active centre of the pandemic.[238] On 19 March 2020, Italy overtook China as the country with the most deaths.[239] By 26 March, the United States had overtaken China and Italy with the highest number of confirmed cases in the world.[240] Research on coronavirus genomes indicates the majority of COVID-19 cases in New York came from European travellers, rather than directly from China or any other Asian country.[241] Retesting of prior samples found a person in France who had the virus on 27 December 2019[242][243] and a person in the United States who died from the disease on 6 February 2020.[244]
On 11 June 2020, after 55 days without a locally transmitted case,[245] Beijing reported the first COVID-19 case, followed by two more cases on 12 June.[246] By 15 June, 79 cases were officially confirmed.[247] Most of these patients went to Xinfadi Wholesale Market.[245][248]
Epidemiology
Several measures are commonly used to quantify mortality.[249] These numbers vary by region and over time and are influenced by the volume of testing, healthcare system quality, treatment options, time since the initial outbreak, and population characteristics such as age, sex, and overall health.[250]
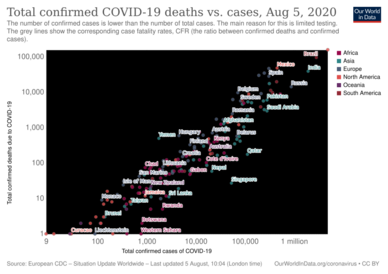
The death-to-case ratio reflects the number of deaths divided by the number of diagnosed cases within a given time interval. Based on Johns Hopkins University statistics, the global death-to-case ratio is 1.90% (5,425,817/285,043,163) as of 30 December 2021.[8] The number varies by region.[251]
Other measures include the case fatality rate (CFR), which reflects the percent of diagnosed individuals who die from a disease, and the infection fatality rate (IFR), which reflects the percent of infected individuals (diagnosed and undiagnosed) who die from a disease. These statistics are not time-bound and follow a specific population from infection through case resolution. Many academics have attempted to calculate these numbers for specific populations.[252]
Outbreaks have occurred in prisons due to crowding and an inability to enforce adequate social distancing.[253][254] In the United States, the prisoner population is aging and many of them are at high risk for poor outcomes from COVID‑19 due to high rates of coexisting heart and lung disease, and poor access to high-quality healthcare.[253]
Infection fatality rate
Infection fatality rate (or infection fatality ratio) is distinguished from case fatality rate. The case fatality rate ("CFR") for a disease is the proportion of deaths from the disease compared to the total number of people diagnosed with the disease (within a certain period of time). The infection fatality ratio ("IFR"), in contrast, is the proportion of deaths among all the infected individuals. IFR, unlike CFR, attempts to account for all asymptomatic and undiagnosed infections.
Our World in Data states that, as of 25 March 2020, the infection fatality rate (IFR) for coronavirus cannot be accurately calculated.[257] In February, the World Health Organization reported estimates of IFR between 0.33% and 1%.[258][259] On 2 July, The WHO's Chief Scientist reported that the average IFR estimate presented at a two-day WHO expert forum was about 0.6%.[260][261]
The CDC estimates for planning purposes that the infection fatality rate is 0.65% and that 40% of infected individuals are asymptomatic, suggesting a fatality rate among those who are symptomatic of 1.08% (.65/60) (as of 10 July).[262][263] According to the University of Oxford Centre for Evidence-Based Medicine (CEBM), random antibody testing in Germany suggested a national IFR of 0.37% (0.12% to 0.87%).[264][265][266] To get a better view on the number of people infected, (As of April 2020), initial antibody testing had been carried out, but peer-reviewed scientific analyses had not yet been published.[267][268] On 1 May antibody testing in New York City suggested an IFR of 0.86%.[269]
Firm lower limits of infection fatality rates have been established in a number of locations such as New York City and Bergamo in Italy since the IFR cannot be less than the population fatality rate. As of 10 July, in New York City , with a population of 8.4 million, 23,377 individuals (18,758 confirmed and 4,619 probable) have died with COVID-19 (0.28% of the population).[270] In Bergamo province, 0.57% of the population has died.[271]
Sex differences
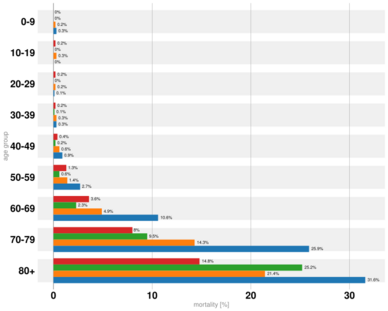
Early reviews of epidemiologic data showed greater impact of the pandemic and a higher mortality rate in men in China and Italy.[272][1][273] The Chinese Center for Disease Control and Prevention reported the death rate was 2.8 percent for men and 1.7 percent for women.[274] Later reviews in June 2020 indicated that there is no significant difference in susceptibility or in CFR between genders.[275][276] One review acknowledges the different mortality rates in Chinese men, suggesting that it may be attributable to lifestyle choices such as smoking and drinking alcohol rather than genetic factors.[277] Sex-based immunological differences, lesser prevalence of smoking in women and men developing co-morbid conditions such as hypertension at a younger age than women could have contributed to the higher mortality in men.[278] In Europe, 57% of the infected people were men and 72% of those died with COVID-19 were men.[279] As of April 2020, the US government is not tracking sex-related data of COVID-19 infections.[280] Research has shown that viral illnesses like Ebola, HIV, influenza and SARS affect men and women differently.[280]
| Percent of infected people who are hospitalized | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Total | |
| Female | 0.2 (0.1–0.3) |
0.6 (0.3–0.9) |
1.1 (0.7–1.8) |
1.6 (0.9–2.4) |
3.2 (1.9–4.9) |
6.2 (3.7–9.6) |
9.6 (5.7–14.8) |
23.6 (14.0–36.4) |
3.2 (1.9–5.0) |
| Male | 0.2 (0.1–0.3) |
0.7 (0.4–1.1) |
1.4 (0.9–2.2) |
1.9 (1.1–3.0) |
3.9 (2.3–6.1) |
8.1 (4.8–12.6) |
13.4 (8.0–20.7) |
45.9 (27.3–70.9) |
4.0 (2.4–6.2) |
| Total | 0.2 (0.1–0.3) |
0.6 (0.4–1.0) |
1.3 (0.8–2.0) |
1.7 (1.0–2.7) |
3.5 (2.1–5.4) |
7.1 (4.2–11.0) |
11.3 (6.7–17.5) |
32.0 (19.0–49.4) |
3.6 (2.1–5.6) |
| Percent of hospitalized people who go to Intensive Care Unit | |||||||||
| 0–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Total | |
| Female | 16.7 (14.4–19.2) |
8.6 (7.5–9.9) |
11.9 (10.9–13.0) |
16.6 (15.6–17.7) |
20.7 (19.8–21.7) |
23.1 (22.2–24.0) |
18.7 (18.0–19.5) |
4.2 (4.0–4.5) |
14.3 (13.9–14.7) |
| Male | 26.9 (23.2–31.0) |
14.0 (12.2–15.9) |
19.2 (17.6–20.9) |
26.9 (25.3–28.5) |
33.4 (32.0–34.8) |
37.3 (36.0–38.6) |
30.2 (29.2–31.3) |
6.8 (6.5–7.2) |
23.1 (22.6–23.6) |
| Total | 22.2 (19.2–25.5) |
11.5 (10.1–13.2) |
15.9 (14.6–17.3) |
22.2 (21.0–23.5) |
27.6 (26.5–28.7) |
30.8 (29.8–31.8) |
24.9 (24.1–25.8) |
5.6 (5.3–5.9) |
19.0 (18.7–19.44) |
| Percent of hospitalized people who die | |||||||||
| 0–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Total | |
| Female | 0.5 (0.2–1.1) |
0.9 (0.5–1.3) |
1.5 (1.2–1.9) |
2.6 (2.3–3.0) |
5.2 (4.8–5.6) |
10.1 (9.5–10.6) |
16.7 (16.0–17.4) |
25.2 (24.4–26.0) |
14.4 (14.0–14.9) |
| Male | 0.7 (0.3–1.5) |
1.3 (0.8–1.9) |
2.2 (1.7–2.7) |
3.8 (3.4–4.4) |
7.6 (7.0–8.2) |
14.8 (14.1–15.6) |
24.6 (23.7–25.6) |
37.1 (36.1–38.2) |
21.22 (20.8–21.7) |
| Total | 0.6 (0.3–1.3) |
1.1 (0.7–1.6) |
1.9 (1.5–2.3) |
3.3 (2.9–3.7) |
6.5 (6.0–7.0) |
12.6 (12.0–13.2) |
21.0 (20.3–21.8) |
31.6 (30.9–32.4) |
18.1 (17.8–18.4) |
| Percent of infected people who die – infection fatality rate (IFR) | |||||||||
| 0–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | Total | |
| Female | 0.001 (<0.001–0.002) |
0.005 (0.002–0.009) |
0.02 (0.01–0.03) |
0.04 (0.02–0.07) |
0.2 (0.1–0.3) |
0.6 (0.4–1.0) |
1.6 (1.0–2.5) |
5.9 (3.5–9.2) |
0.5 (0.3–0.7) |
| Male | 0.001 (<0.001–0.003) |
0.008 (0.004–0.02) |
0.03 (0.02–0.05) |
0.07 (0.04–0.1) |
0.3 (0.2–0.5) |
1.2 (0.7–1.9) |
3.3 (2.0–5.1) |
17.1 (10.1–26.3) |
0.8 (0.5–1.3) |
| Total | 0.001 (<0.001–0.002) |
0.007 (0.003–0.01) |
0.02 (0.01–0.04) |
0.06 (0.03–0.09) |
0.2 (0.1–0.36) |
0.9 (0.5–1.4) |
2.4 (1.4–3.7) |
10.1 (6.0–15.6) |
0.7 (0.4–1.0) |
| Numbers in parentheses are 95% credible intervals for the estimates. | |||||||||
Ethnic differences
In the US, a greater proportion of deaths due to COVID-19 have occurred among African Americans.[282] Structural factors that prevent African Americans from practicing social distancing include their concentration in crowded substandard housing and in "essential" occupations such as public transit and health care. Greater prevalence of lacking health insurance and care and of underlying conditions such as diabetes, hypertension and heart disease also increase their risk of death.[283] Similar issues affect Native American and Latino communities.[282] According to a US health policy non-profit, 34% of American Indian and Alaska Native People (AIAN) non-elderly adults are at risk of serious illness compared to 21% of white non-elderly adults.[284] The source attributes it to disproportionately high rates of many health conditions that may put them at higher risk as well as living conditions like lack of access to clean water.[285] Leaders have called for efforts to research and address the disparities.[286]
In the U.K., a greater proportion of deaths due to COVID-19 have occurred in those of a Black, Asian, and other ethnic minority background.[287][288][289] Several factors such as poverty, poor nutrition and living in overcrowded properties, may have caused this.[citation needed]
Society and culture
Name
During the initial outbreak in Wuhan, China, the virus and disease were commonly referred to as "coronavirus" and "Wuhan coronavirus",[290][291][292] with the disease sometimes called "Wuhan pneumonia".[293][294] In the past, many diseases have been named after geographical locations, such as the Spanish flu,[295] Middle East Respiratory Syndrome, and Zika virus.[296]
In January 2020, the World Health Organisation recommended 2019-nCov[297] and 2019-nCoV acute respiratory disease[298] as interim names for the virus and disease per 2015 guidance and international guidelines against using geographical locations (e.g. Wuhan, China), animal species or groups of people in disease and virus names to prevent social stigma.[299][300][301]
The official names COVID‑19 and SARS-CoV-2 were issued by the WHO on 11 February 2020.[302] WHO chief Tedros Adhanom Ghebreyesus explained: CO for corona, VI for virus, D for disease and 19 for when the outbreak was first identified (31 December 2019).[303] The WHO additionally uses "the COVID‑19 virus" and "the virus responsible for COVID‑19" in public communications.[302]
Misinformation
After the initial outbreak of COVID‑19, misinformation and disinformation regarding the origin, scale, prevention, treatment, and other aspects of the disease rapidly spread online.[304][305][306]
Other health issues
The pandemic has had many impacts on global health beyond those caused by the COVID-19 disease itself. It has led to a reduction in hospital visits for other reasons. There have been 38% fewer hospital visits for heart attack symptoms in the United States and 40% fewer in Spain.[307] The head of cardiology at the University of Arizona said, "My worry is some of these people are dying at home because they're too scared to go to the hospital."[308] There is also concern that people with strokes and appendicitis are not seeking timely treatment.[308] Shortages of medical supplies have impacted people with various conditions.[309] In several countries there has been a marked reduction of spread of sexually transmitted infections, including HIV, attributable to COVID-19 quarantines and social distancing measures.[310][311] Similarly, in some places, rates of transmission of influenza and other respiratory viruses significantly decreased during the pandemic.[312][313][314] The pandemic has also negatively impacted mental health globally, including increased loneliness resulting from social distancing.[315]
Other animals
Humans appear to be capable of spreading the virus to some other animals. A domestic cat in Liège, Belgium, tested positive after it started showing symptoms (diarrhoea, vomiting, shortness of breath) a week later than its owner, who was also positive.[316] Tigers and lions at the Bronx Zoo in New York, United States, tested positive for the virus and showed symptoms of COVID‑19, including a dry cough and loss of appetite.[317] Minks at two farms in the Netherlands also tested positive for COVID-19.[318]
A study on domesticated animals inoculated with the virus found that cats and ferrets appear to be "highly susceptible" to the disease, while dogs appear to be less susceptible, with lower levels of viral replication. The study failed to find evidence of viral replication in pigs, ducks, and chickens.[319]
In March 2020, researchers from the University of Hong Kong have shown that Syrian hamsters could be a model organism for COVID-19 research.[320]
Research
No medication or vaccine is approved with the specific indication to treat the disease.[321] International research on vaccines and medicines in COVID‑19 is underway by government organisations, academic groups, and industry researchers.[322][323] In March, the World Health Organisation initiated the "Solidarity Trial" to assess the treatment effects of four existing antiviral compounds with the most promise of efficacy.[324] The World Health Organization suspended hydroxychloroquine from its global drug trials for COVID-19 treatments on 26 May 2020 due to safety concerns. It had previously enrolled 3,500 patients from 17 countries in the Solidarity Trial.[325] France, Italy and Belgium also banned the use of hydroxychloroquine as a COVID-19 treatment.[326]
There has been a great deal of COVID-19 research, involving accelerated research processes and publishing shortcuts to meet the global demand. To minimise the harm from misinformation, medical professionals and the public are advised to expect rapid changes to available information, and to be attentive to retractions and other updates.[327]
Vaccine
There is no available vaccine, but various agencies are actively developing vaccine candidates. Previous work on SARS-CoV is being used because both SARS-CoV and SARS-CoV-2 use the ACE2 receptor to enter human cells.[328] Six vaccination strategies are being investigated. Four of these, as of early July 2020, are being tested in clinical trials.[329] First, researchers aim to build a whole virus vaccine. The use of such inactive virus aims to elicit a prompt immune response of the human body to a new infection with COVID‑19. A second strategy, subunit vaccines, aims to create a vaccine that sensitises the immune system to certain subunits of the virus. In the case of SARS-CoV-2, such research focuses on the S-spike protein that helps the virus intrude the ACE2 enzyme receptor. A third strategy is that of the nucleic acid vaccines (DNA or RNA vaccines, a novel technique for creating a vaccination). Fourthly, scientists are attempting to use viral vectors to deliver the SARS-CoV-2 antigen gene into the cell.[330] These can be replicating or non-replicating. As of early July 2020, only non-replicating viral vectors are in clinical trials. Viral vectors in clinical trials include Chimpanzee Adenovirus 63,[330] Adenovirus type-5,[329] and Adenovirus type-26.[331] Scientists are also working to develop an attenuated COVID-19 vaccine and a COVID-19 vaccine using virus-like particles, but these are still in preclinical research.[329] Experimental vaccines from any of these strategies would have to be tested for safety and efficacy.[332]
Antibody-dependent enhancement has been suggested as a potential challenge for vaccine development for SARS-COV-2, but this is controversial.[333]
Medications
At least 29 phase II–IV efficacy trials in COVID‑19 were concluded in March 2020, or scheduled to provide results in April from hospitals in China.[334][335] There are more than 300 active clinical trials underway as of April 2020.[129] Seven trials were evaluating already approved treatments, including four studies on hydroxychloroquine or chloroquine.[335] Repurposed antiviral drugs make up most of the research, with nine phase III trials on remdesivir across several countries due to report by the end of April.[334][335] Other candidates in trials include vasodilators, corticosteroids, immune therapies, lipoic acid, bevacizumab, and recombinant angiotensin-converting enzyme 2.[335]
The COVID‑19 Clinical Research Coalition has goals to 1) facilitate rapid reviews of clinical trial proposals by ethics committees and national regulatory agencies, 2) fast-track approvals for the candidate therapeutic compounds, 3) ensure standardised and rapid analysis of emerging efficacy and safety data and 4) facilitate sharing of clinical trial outcomes before publication.[336][337]
Several existing medications are being evaluated for the treatment of COVID‑19,[321] including remdesivir, chloroquine, hydroxychloroquine, lopinavir/ritonavir, and lopinavir/ritonavir combined with interferon beta.[324][338] There is tentative evidence for efficacy by remdesivir, and on 1 May 2020, the United States Food and Drug Administration (FDA) gave the drug an emergency use authorization for people hospitalized with severe COVID‑19.[339] Phase III clinical trials for several drugs are underway in several countries, including the US, China, and Italy.[321][334][340]
There are mixed results as of 3 April 2020 as to the effectiveness of hydroxychloroquine as a treatment for COVID‑19, with some studies showing little or no improvement.[341][342] One study has shown an association between hydroxychloroquine or chloroquine use with higher death rates along with other side effects.[343][344] A retraction of this study by its authors was published by The Lancet on 4 June 2020.[345] The studies of chloroquine and hydroxychloroquine with or without azithromycin have major limitations that have prevented the medical community from embracing these therapies without further study.[129] On 15 June 2020, the FDA updated the fact sheets for the emergency use authorization of remdesivir to warn that using chloroquine or hydroxychloroquine with remdesivir may reduce the antiviral activity of remdesivir.[346]
In June, initial results from a randomised trial in the United Kingdom showed that dexamethasone reduced mortality by one third for patients who are critically ill on ventilators and one fifth for those receiving supplemental oxygen.[347] Because this is a well tested and widely available treatment this was welcomed by the WHO that is in the process of updating treatment guidelines to include dexamethasone or other steroids.[348][349] Based on those preliminary results, dexamethasone treatment has been recommended by the National Institutes of Health for patients with COVID-19 who are mechanically ventilated or who require supplemental oxygen but not in patients with COVID-19 who do not require supplemental oxygen.[350]
Cytokine storm
A cytokine storm can be a complication in the later stages of severe COVID‑19. There is preliminary evidence that hydroxychloroquine may be useful in controlling cytokine storms in late-phase severe forms of the disease.[351]
Tocilizumab has been included in treatment guidelines by China's National Health Commission after a small study was completed.[352][353] It is undergoing a phase 2 non-randomised trial at the national level in Italy after showing positive results in people with severe disease.[354][355] Combined with a serum ferritin blood test to identify a cytokine storm (also called cytokine storm syndrome, not to be confused with cytokine release syndrome), it is meant to counter such developments, which are thought to be the cause of death in some affected people.[356][357][358] The interleukin-6 receptor antagonist was approved by the Food and Drug Administration (FDA) to undergo a phase III clinical trial assessing its effectiveness on COVID‑19 based on retrospective case studies for the treatment of steroid-refractory cytokine release syndrome induced by a different cause, CAR T cell therapy, in 2017.[359] To date,[when?] there is no randomised, controlled evidence that tocilizumab is an efficacious treatment for CRS. Prophylactic tocilizumab has been shown to increase serum IL-6 levels by saturating the IL-6R, driving IL-6 across the blood-brain barrier, and exacerbating neurotoxicity while having no effect on the incidence of CRS.[360]
Lenzilumab, an anti-GM-CSF monoclonal antibody, is protective in murine models for CAR T cell-induced CRS and neurotoxicity and is a viable therapeutic option due to the observed increase of pathogenic GM-CSF secreting T-cells in hospitalised patients with COVID‑19.[361]
The Feinstein Institute of Northwell Health announced in March a study on "a human antibody that may prevent the activity" of IL-6.[362]
Passive antibodies
Transferring purified and concentrated antibodies produced by the immune systems of those who have recovered from COVID‑19 to people who need them is being investigated as a non-vaccine method of passive immunisation.[363][364] The safety and effectiveness of convalescent plasma as a treatment option requires further research.[364] This strategy was tried for SARS with inconclusive results.[363] Viral neutralisation is the anticipated mechanism of action by which passive antibody therapy can mediate defence against SARS-CoV-2. The spike protein of SARS-CoV-2 is the primary target for neutralizing antibodies.[365] It has been proposed that selection of broad-neutralizing antibodies against SARS-CoV-2 and SARS-CoV might be useful for treating not only COVID-19 but also future SARS-related CoV infections.[365] Other mechanisms, however, such as antibody-dependent cellular cytotoxicity and/or phagocytosis, may be possible.[363] Other forms of passive antibody therapy, for example, using manufactured monoclonal antibodies, are in development.[363] Production of convalescent serum, which consists of the liquid portion of the blood from recovered patients and contains antibodies specific to this virus, could be increased for quicker deployment.[366]
See also
- Coronavirus diseases, a group of closely related syndromes
- Coronavirus recession
- Decoding COVID-19, 2020 PBS film documentary about the 2019–2020 COVID-19 pandemic
- Disease X, a WHO term
- Li Wenliang, a doctor who died of COVID-19 after raising awareness of its spread
- List of unproven methods against COVID-19
Notes
References
- ↑ 1.0 1.1 1.2 "Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study". Lancet 395 (10223): 507–513. February 2020. doi:10.1016/S0140-6736(20)30211-7. PMID 32007143.
- ↑ "Novel Coronavirus Pneumonia (COVID-19) Progression Course in 17 Discharged Patients: Comparison of Clinical and Thin-Section CT Features During Recovery". Clinical Infectious Diseases. March 2020. doi:10.1093/cid/ciaa271. PMID 32227091.
- ↑ "Special Act for Prevention, Relief and Revitalization Measures for Severe Pneumonia with Novel Pathogens–Article Content–Laws & Regulations Database of The Republic of China". https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=L0050039.
- ↑ "Covid-19, n.". https://oed.com/view/Entry/88575495.
- ↑ 5.0 5.1 5.2 5.3 "Symptoms of Coronavirus". 13 May 2020. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 6.14 6.15 "Q&A on coronaviruses (COVID-19)". World Health Organization. 17 April 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/q-a-coronaviruses.
- ↑ 7.0 7.1 "Quarantine alone or in combination with other public health measures to control COVID-19: a rapid review". The Cochrane Database of Systematic Reviews 4: CD013574. April 2020. doi:10.1002/14651858.CD013574. PMID 32267544.
- ↑ 8.0 8.1 8.2 8.3 "COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU)". Johns Hopkins University. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6.
- ↑ "Coronavirus disease 2019 (COVID-19)—Symptoms and causes". https://www.mayoclinic.org/diseases-conditions/coronavirus/symptoms-causes/syc-20479963.
- ↑ 10.0 10.1 10.2 "The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China". International Journal of Infectious Diseases 91: 264–266. February 2020. doi:10.1016/j.ijid.2020.01.009. PMID 31953166.
- ↑ 11.0 11.1 "WHO Director-General's opening remarks at the media briefing on COVID-19". World Health Organization (WHO) (Press release). 11 March 2020. Archived from the original on 11 March 2020. Retrieved 12 March 2020.
- ↑ 12.0 12.1 Ma, Josephine (13 March 2020). "Coronavirus: China's first confirmed Covid-19 case traced back to November 17". https://www.scmp.com/news/china/society/article/3074991/coronavirus-chinas-first-confirmed-covid-19-case-traced-back.
- ↑ 13.0 13.1 13.2 Grant, Michael C.; Geoghegan, Luke; Arbyn, Marc; Mohammed, Zakaria; McGuinness, Luke; Clarke, Emily L.; Wade, Ryckie G.; Hirst, Jennifer A. (23 June 2020). "The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries". PLOS ONE 15 (6): e0234765. doi:10.1371/journal.pone.0234765. PMID 32574165.
- ↑ 14.0 14.1 Hopkins, Claire. "Loss of sense of smell as marker of COVID-19 infection". https://www.entuk.org/loss-sense-smell-marker-covid-19-infection.
- ↑ "The pathogenesis and treatment of the 'Cytokine Storm' in COVID-19". J. Infect. 80 (6): 607–613. June 2020. doi:10.1016/j.jinf.2020.03.037. PMID 32283152.
- ↑ 16.0 16.1 "Care for Critically Ill Patients With COVID-19". JAMA 323 (15): 1499. March 2020. doi:10.1001/jama.2020.3633. PMID 32159735.
- ↑ "Features, Evaluation and Treatment Coronavirus (COVID-19)". StatPearls. Treasure Island (FL): StatPearls Publishing. 2020. http://www.ncbi.nlm.nih.gov/books/NBK554776/. Retrieved 18 March 2020.
- ↑ "COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-up". Journal of the American College of Cardiology 75 (23): 2950–2973. April 2020. doi:10.1016/j.jacc.2020.04.031. PMID 32311448.
- ↑ "The COVID-19 epidemic". Tropical Medicine & International Health 25 (3): 278–280. March 2020. doi:10.1111/tmi.13383. PMID 32052514.
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 20.7 20.8 20.9 "How COVID-19 Spreads". 2 April 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html.
- ↑ "Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19". JAMA. March 2020. doi:10.1001/jama.2020.4756. PMID 32215590.
- ↑ 22.00 22.01 22.02 22.03 22.04 22.05 22.06 22.07 22.08 22.09 22.10 "Q & A on COVID-19". https://www.ecdc.europa.eu/en/covid-19/questions-answers.
- ↑ "The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission". Proceedings of the National Academy of Sciences of the United States of America 117 (22): 11875–11877. June 2020. doi:10.1073/pnas.2006874117. PMID 32404416.
- ↑ 24.0 24.1 24.2 "Q&A: How is COVID-19 transmitted?" (in en). https://www.who.int/news-room/q-a-detail/q-a-how-is-covid-19-transmitted.
- ↑ 25.0 25.1 "Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19)". 11 February 2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html.
- ↑ 26.0 26.1 26.2 26.3 26.4 "Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients". AJR. American Journal of Roentgenology 215 (1): 87–93. March 2020. doi:10.2214/AJR.20.23034. PMID 32174129.
- ↑ 27.0 27.1 "ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection". 22 March 2020. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection.
- ↑ "Advice for public". https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public.
- ↑ "Guidance on social distancing for everyone in the UK". https://www.gov.uk/government/publications/covid-19-guidance-on-social-distancing-and-for-vulnerable-people/guidance-on-social-distancing-for-everyone-in-the-uk-and-protecting-older-people-and-vulnerable-adults.
- ↑ 30.0 30.1 30.2 30.3 "Recommendations for Cloth Face Covers". 3 April 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover.html.
- ↑ "Rational use of face masks in the COVID-19 pandemic". The Lancet. Respiratory Medicine 8 (5): 434–436. May 2020. doi:10.1016/S2213-2600(20)30134-X. PMID 32203710.
- ↑ 32.0 32.1 32.2 "What to Do if You Are Sick". 5 April 2020. https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/steps-when-sick.html.
- ↑ "When and how to use masks". https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks.
- ↑ "How to Protect Yourself & Others". U.S. Centers for Disease Control and Prevention (CDC). 8 April 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html.
- ↑ "Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)". https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov).
- ↑ "Hundreds of evacuees to be held on bases in California; Hong Kong and Taiwan restrict travel from mainland China". The Washington Post. 6 February 2020. https://www.washingtonpost.com/world/asia_pacific/coronavirus-china-live-updates/2020/02/05/114ced8a-479c-11ea-bc78-8a18f7afcee7_story.html.
- ↑ "WHO Situation Report #87". 16 April 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200416-sitrep-87-covid-19.pdf?sfvrsn=9523115a_2.
- ↑ "Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19)". 6 April 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html.
- ↑ "Coronavirus Disease (COVID-19): A primer for emergency physicians". The American Journal of Emergency Medicine. March 2020. doi:10.1016/j.ajem.2020.03.036. PMID 32265065.
- ↑ "Current epidemiological and clinical features of COVID-19; a global perspective from China". The Journal of Infection 81 (1): 1–9. April 2020. doi:10.1016/j.jinf.2020.04.011. PMID 32315723.
- ↑ Guan, Wei-jie; Ni, Zheng-yi; Hu, Yu; Liang, Wen-hua; Ou, Chun-quan; He, Jian-xing; Liu, Lei; Shan, Hong et al. (30 April 2020). "Clinical Characteristics of Coronavirus Disease 2019 in China". New England Journal of Medicine (Massachusetts Medical Society) 382 (18): 1708–1720. doi:10.1056/nejmoa2002032. ISSN 0028-4793. PMID 32109013.
- ↑ Hessen, Margaret Trexler (27 January 2020). "Novel Coronavirus Information Center: Expert guidance and commentary". https://www.elsevier.com/connect/coronavirus-information-center.
- ↑ Wei XS, Wang X, Niu YR, Ye LL, Peng WB, Wang ZH, et al. (February 2020). "Clinical Characteristics of SARS-CoV-2 Infected Pneumonia with Diarrhea". SSRN 3546120.
- ↑ 44.0 44.1 44.2 "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China". Lancet 395 (10223): 497–506. February 2020. doi:10.1016/S0140-6736(20)30183-5. PMID 31986264.
- ↑ "Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges". International Journal of Antimicrobial Agents 55 (3): 105924. March 2020. doi:10.1016/j.ijantimicag.2020.105924. PMID 32081636.
- ↑ 46.0 46.1 46.2 Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19) (Report). World Health Organization (WHO). 16–24 February 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf. Retrieved 21 March 2020.
- ↑ 47.0 47.1 "COVID-19 and the cardiovascular system". Nature Reviews. Cardiology 17 (5): 259–260. May 2020. doi:10.1038/s41569-020-0360-5. PMID 32139904.
- ↑ "Smell and taste dysfunction in patients with COVID-19". The Lancet. Infectious Diseases. April 2020. doi:10.1016/S1473-3099(20)30293-0. PMID 32304629. PMC 7159875. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30293-0/fulltext#%20.
- ↑ "Symptoms of Coronavirus". U.S. Centers for Disease Control and Prevention (CDC). 27 April 2020. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html.
- ↑ "Sixty seconds on ... anosmia". BMJ 368: m1202. March 2020. doi:10.1136/bmj.m1202. PMID 32209546.
- ↑ Coronavirus disease 2019 (COVID-19): situation report, 29 (Report). World Health Organization. 19 February 2020.
- ↑ Rapid Expert Consultation Update on SARS-CoV-2 Surface Stability and Incubation for the COVID-19 Pandemic. 27 March 2020. doi:10.17226/25763. ISBN 978-0-309-67610-6. https://www.nap.edu/read/25763/chapter/1. Retrieved 18 May 2020.
- ↑ "Interim Guidance: Public Health Management of cases and contacts associated with novel coronavirus (COVID-19) in the community". 15 May 2020. http://www.bccdc.ca/resource-gallery/Documents/Guidelines%20and%20Forms/Guidelines%20and%20Manuals/Epid/CD%20Manual/Chapter%201%20-%20CDC/2019-nCoV-Interim_Guidelines.pdf.
- ↑ "Rapid Review of the literature: Assessing the infection prevention and control measures for the prevention and management of COVID-19 in health and care settings". 20 April 2020. https://hpspubsrepo.blob.core.windows.net/hps-website/nss/2985/documents/1_covid-19-rapid-review-ipc-for-covid-19.pdf.
- ↑ "The Early Natural History of SARS-CoV-2 Infection: Clinical Observations From an Urban, Ambulatory COVID-19 Clinic". Mayo Clinic Proceedings 95 (6): 1124–1126. June 2020. doi:10.1016/j.mayocp.2020.04.010. PMID 32451119.
- ↑ 56.0 56.1 Gao, Zhiru; Xu, Yinghui; Sun, Chao; Wang, Xu; Guo, Ye; Qiu, Shi; Ma, Kewei (15 May 2020). "A systematic review of asymptomatic infections with COVID-19". Journal of Microbiology, Immunology and Infection. doi:10.1016/j.jmii.2020.05.001. ISSN 1684-1182. PMID 32425996. PMC 7227597. https://www.sciencedirect.com/science/article/pii/S1684118220301134. Retrieved 13 June 2020.
- ↑ "Clinical Questions about COVID-19: Questions and Answers". 11 February 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/faq.html.
- ↑ "Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths". Journal of Microbiology, Immunology, and Infection = Wei Mian Yu Gan Ran Za Zhi 53 (3): 404–412. March 2020. doi:10.1016/j.jmii.2020.02.012. PMID 32173241.
- ↑ "Evidence Supporting Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 While Presymptomatic or Asymptomatic". Emerging Infectious Diseases 26 (7). May 2020. doi:10.3201/eid2607.201595. PMID 32364890.
- ↑ Feuer, William; Higgins-Dunn, Noah (8 June 2020). "Asymptomatic spread of coronavirus is 'very rare,' WHO says". https://www.cnbc.com/2020/06/08/asymptomatic-coronavirus-patients-arent-spreading-new-infections-who-says.html.
- ↑ Oran, DP; Topol, EJ (3 June 2020). "Prevalence of Asymptomatic SARS-CoV-2 Infection: A Narrative Review.". Annals of Internal Medicine. doi:10.7326/M20-3012. PMID 32491919.
- ↑ "High SARS-CoV-2 Attack Rate Following Exposure at a Choir Practice—Skagit County, Washington, March 2020". MMWR Morb. Mortal. Wkly. Rep. 69 (19): 606–610. May 2020. doi:10.15585/mmwr.mm6919e6. PMID 32407303. https://www.cdc.gov/mmwr/volumes/69/wr/pdfs/mm6919e6-H.pdf.
- ↑ Jean François Gehanno, Vincent Bonneterre, Pascal Andujar, Jean-Claude Pairon, Christophe Paris, Audrey Petit, Catherine Verdun-Esquer, Alexis Descatha, Quentin V Durand-Moreau, Patrick Brochard (30 Jun 2020). How should data on airborne transmission of SARS-CoV-2 change occupational health guidelines?. Occupational and Environmental Medicine.
- ↑ Lewis, Dyani (2020-07-08). "Mounting evidence suggests coronavirus is airborne — but health advice has not caught up" (in en). Nature. doi:10.1038/d41586-020-02058-1. https://www.nature.com/articles/d41586-020-02058-1.
- ↑ "Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review". PLOS ONE 7 (4): e35797. 2012. doi:10.1371/journal.pone.0035797. PMID 22563403. Bibcode: 2012PLoSO...735797T.
- ↑ "New coronavirus stable for hours on surfaces". National Institutes of Health. 17 March 2020. https://www.nih.gov/news-events/news-releases/new-coronavirus-stable-hours-surfaces.
- ↑ "Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1". New England Journal of Medicine 382 (16): 1564–1567. April 2020. doi:10.1056/NEJMc2004973. PMID 32182409.
- ↑ "Household cleaners and disinfectants can cause health problems when not used properly.". U.S. Centers for Disease Control and Prevention (CDC). 24 April 2020. https://twitter.com/cdcgov/status/1253742258853199872?lang=en.
- ↑ 69.0 69.1 "Consistent detection of 2019 novel coronavirus in saliva". Clinical Infectious Diseases (Oxford University Press). February 2020. doi:10.1093/cid/ciaa149. PMID 32047895.
- ↑ "COVID-19 and Our Communities–ACON–We are a New South Wales based health promotion organisation specialising in HIV prevention, HIV support and lesbian, gay, bisexual, transgender and intersex (LGBTI) health". Acon.org.au. https://www.acon.org.au/what-we-are-here-for/covid19/.
- ↑ "Sex and Coronavirus Disease 2019 (COVID-19)". 27 March 2020. https://www1.nyc.gov/assets/doh/downloads/pdf/imm/covid-sex-guidance.pdf.
- ↑ Bingmann, Annika (22 May 2020). "Latest findings by Ulm virologists – New coronavirus detected in breast milk". https://www.uni-ulm.de/en/med/faculty/med-detailseiten/news-detail/article/moeglicher-uebertragungsweg-von-sars-cov-2-erstmals-neues-coronavirus-in-muttermilch-nachgewiesen-1/.
- ↑ "Detection of SARS-CoV-2 in human breastmilk" (in English). Lancet 0 (10239): 1757–1758. May 2020. doi:10.1016/S0140-6736(20)31181-8. PMID 32446324. PMC 7241971. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31181-8/fulltext.
- ↑ "Novel Coronavirus—Information for Clinicians". https://www.health.gov.au/sites/default/files/documents/2020/03/coronavirus-covid-19-information-for-clinicians.pdf.
- ↑ "Outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): increased transmission beyond China—fourth update". European Centre for Disease Prevention and Control. 14 February 2020. https://www.ecdc.europa.eu/sites/default/files/documents/SARS-CoV-2-risk-assessment-14-feb-2020.pdf.
- ↑ 76.0 76.1 "The proximal origin of SARS-CoV-2". Nature Medicine 26 (4): 450–452. April 2020. doi:10.1038/s41591-020-0820-9. PMID 32284615.
- ↑ "A Novel Coronavirus from Patients with Pneumonia in China, 2019". New England Journal of Medicine 382 (8): 727–733. February 2020. doi:10.1056/NEJMoa2001017. PMID 31978945.
- ↑ "Mystery deepens over animal source of coronavirus". Nature 579 (7797): 18–19. March 2020. doi:10.1038/d41586-020-00548-w. PMID 32127703. Bibcode: 2020Natur.579...18C.
- ↑ "The pivotal link between ACE2 deficiency and SARS-CoV-2 infection". European Journal of Internal Medicine 76: 14–20. June 2020. doi:10.1016/j.ejim.2020.04.037. PMID 32336612.
- ↑ "Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses". Nature Microbiology 5 (4): 562–569. April 2020. doi:10.1038/s41564-020-0688-y. PMID 32094589.
- ↑ "Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target". Intensive Care Medicine 46 (4): 586–590. April 2020. doi:10.1007/s00134-020-05985-9. PMID 32125455.
- ↑ 82.0 82.1 "High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa". International Journal of Oral Science 12 (1): 8. February 2020. doi:10.1038/s41368-020-0074-x. PMID 32094336.
- ↑ "Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics". Drug Development Research. March 2020. doi:10.1002/ddr.21656. PMID 32129518.
- ↑ "The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients". Journal of Medical Virology 92 (6): 552–555. February 2020. doi:10.1002/jmv.25728. PMID 32104915.
- ↑ "Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms". ACS Chemical Neuroscience 11 (7): 995–998. April 2020. doi:10.1021/acschemneuro.0c00122. PMID 32167747.
- ↑ "COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission". Gastroenterology 158 (6): 1518–1519. May 2020. doi:10.1053/j.gastro.2020.02.054. PMID 32142785.
- ↑ "Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis". The Journal of Pathology 203 (2): 631–7. June 2004. doi:10.1002/path.1570. PMID 15141377.
- ↑ 88.0 88.1 88.2 "COVID-19 and the cardiovascular system". Nature Reviews. Cardiology 17 (5): 259–260. May 2020. doi:10.1038/s41569-020-0360-5. PMID 32139904.
- ↑ "Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China". JAMA 323 (11): 1061–1069. February 2020. doi:10.1001/jama.2020.1585. PMID 32031570.
- ↑ "ACE2: from vasopeptidase to SARS virus receptor". Trends in Pharmacological Sciences 25 (6): 291–4. June 2004. doi:10.1016/j.tips.2004.04.001. PMID 15165741. PMC 7119032. https://www.cell.com/trends/pharmacological-sciences/abstract/S0165-6147(04)00097-5.
- ↑ "Incidence of thrombotic complications in critically ill ICU patients with COVID-19". Thrombosis Research 191: 145–147. April 2020. doi:10.1016/j.thromres.2020.04.013. PMID 32291094.
- ↑ "Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia". Journal of Thrombosis and Haemostasis 18 (6): 1421–1424. April 2020. doi:10.1111/jth.14830. PMID 32271988.
- ↑ 93.0 93.1 Wadman, Meredith (April 2020). "How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes". Science. doi:10.1126/science.abc3208.
- ↑ Coronavirus: Kidney Damage Caused by COVID-19, Johns Hopkins Medicine, C. John Sperati, updated 14 May 2020.
- ↑ 95.0 95.1 "COVID-19 Autopsies, Oklahoma, USA". American Journal of Clinical Pathology 153 (6): 725–733. May 2020. doi:10.1093/ajcp/aqaa062. PMID 32275742.
- ↑ "The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality". International Journal of Antimicrobial Agents 55 (5): 105954. March 2020. doi:10.1016/j.ijantimicag.2020.105954. PMID 32234467.
- ↑ "CDC Tests for 2019-nCoV". 5 February 2020. https://www.cdc.gov/coronavirus/2019-ncov/about/testing.html.
- ↑ "Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases". https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117.
- ↑ "2019 Novel Coronavirus (2019-nCoV) Situation Summary". 30 January 2020. https://www.cdc.gov/coronavirus/2019-ncov/summary.html.
- ↑ "Real-Time RT-PCR Panel for Detection 2019-nCoV". 29 January 2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-detection-instructions.html.
- ↑ "Curetis Group Company Ares Genetics and BGI Group Collaborate to Offer Next-Generation Sequencing and PCR-based Coronavirus (2019-nCoV) Testing in Europe". 30 January 2020. https://www.globenewswire.com/news-release/2020/01/30/1977226/0/en/Curetis-Group-Company-Ares-Genetics-and-BGI-Group-Collaborate-to-Offer-Next-Generation-Sequencing-and-PCR-based-Coronavirus-2019-nCoV-Testing-in-Europe.html.
- ↑ Brueck, Hilary (30 January 2020). "There's only one way to know if you have the coronavirus, and it involves machines full of spit and mucus". https://www.businessinsider.com/how-to-know-if-you-have-the-coronavirus-pcr-test-2020-1.
- ↑ "Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases". https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117.
- ↑ "New SARS-like virus in China triggers alarm". Science 367 (6475): 234–235. January 2020. doi:10.1126/science.367.6475.234. PMID 31949058. Bibcode: 2020Sci...367..234C. https://mcb.uconn.edu/wp-content/uploads/sites/2341/2020/01/WuhanScience24Jan2020.pdf. Retrieved 11 February 2020.
- ↑ "Severe acute respiratory syndrome coronavirus 2 data hub". https://www.ncbi.nlm.nih.gov/labs/virus/vssi/#/virus?SeqType_s=Nucleotide&VirusLineage_ss=Wuhan%20seafood%20market%20pneumonia%20virus,%20taxid:2697049.
- ↑ "Developing antibody tests for SARS-CoV-2". Lancet 395 (10230): 1101–1102. April 2020. doi:10.1016/s0140-6736(20)30788-1. PMID 32247384.
- ↑ Vogel, Gretchen (March 2020). "New blood tests for antibodies could show true scale of coronavirus pandemic". Science. doi:10.1126/science.abb8028.
- ↑ "Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel Coronavirus (2019-nCoV): A Systematic Review". Journal of Clinical Medicine 9 (3): 623. February 2020. doi:10.3390/jcm9030623. PMID 32110875.
- ↑ Deeks, Jonathan J.; Dinnes, Jacqueline; Takwoingi, Yemisi; Davenport, Clare; Spijker, René; Taylor-Phillips, Sian; Adriano, Ada; Beese, Sophie et al. (June 25, 2020). "Antibody tests for identification of current and past infection with SARS-CoV-2". The Cochrane Database of Systematic Reviews 6: CD013652. doi:10.1002/14651858.CD013652. ISSN 1469-493X. PMID 32584464. https://www.ncbi.nlm.nih.gov/pubmed/32584464.
- ↑ AFP News Agency (11 April 2020). "How false negatives are complicating COVID-19 testing". Al Jazeera website Retrieved 12 April 2020.
- ↑ "Coronavirus (COVID-19) Update: FDA Issues first Emergency Use Authorization for Point of Care Diagnostic" (Press release). U.S. Food and Drug Administration (FDA). 21 March 2020. Archived from the original on 21 March 2020. Retrieved 22 March 2020.
- ↑ Struyf, Thomas; Deeks, Jonathan J.; Dinnes, Jacqueline; Takwoingi, Yemisi; Davenport, Clare; Leeflang, Mariska Mg; Spijker, René; Hooft, Lotty et al. (2020). "Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease". The Cochrane Database of Systematic Reviews 7: CD013665. doi:10.1002/14651858.CD013665. ISSN 1469-493X. PMID 32633856. https://www.ncbi.nlm.nih.gov/pubmed/32633856.
- ↑ "A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version)". Military Medical Research 7 (1): 4. February 2020. doi:10.1186/s40779-020-0233-6. PMID 32029004.
- ↑ "COVID-19 pneumonia: what has CT taught us?". The Lancet. Infectious Diseases 20 (4): 384–385. April 2020. doi:10.1016/S1473-3099(20)30134-1. PMID 32105641. PMC 7128449. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30134-1/fulltext. Retrieved 13 March 2020.
- ↑ "ICD-10 Version:2019". 2019. https://icd.who.int/browse10/2019/en#/U07.1. "U07.2—COVID-19, virus not identified—COVID-19 NOS—Use this code when COVID-19 is diagnosed clinically or epidemiologically but laboratory testing is inconclusive or not available. Use additional code, if desired, to identify pneumonia or other manifestations"
- ↑ "Autopsy in suspected COVID-19 cases". Journal of Clinical Pathology 73 (5): 239–242. May 2020. doi:10.1136/jclinpath-2020-206522. PMID 32198191.
- ↑ "[A pathological report of three COVID-19 cases by minimally invasive autopsies]" (in Chinese). Zhonghua Bing Li Xue Za Zhi = Chinese Journal of Pathology 49 (5): 411–417. March 2020. doi:10.3760/cma.j.cn112151-20200312-00193. PMID 32172546.
- ↑ "Exuberant Plasmocytosis in Bronchoalveolar Lavage Specimen of the First Patient Requiring Extracorporeal Membrane Oxygenation for SARS-CoV-2 in Europe". Journal of Thoracic Oncology 15 (5): e65–e66. May 2020. doi:10.1016/j.jtho.2020.03.008. PMID 32194247.
- ↑ "Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia". Journal of Thrombosis and Haemostasis 18 (4): 786–787. April 2020. doi:10.1111/jth.14781. PMID 32212240.
- ↑ "Leukoerythroblastic reaction in a patient with COVID-19 infection". American Journal of Hematology. March 2020. doi:10.1002/ajh.25793. PMID 32212392.
- ↑ Maier, Benjamin F.; Brockmann, Dirk (15 May 2020). "Effective containment explains subexponential growth in recent confirmed COVID-19 cases in China". Science 368 (6492): 742–746. doi:10.1126/science.abb4557. PMID 32269067. Bibcode: 2020Sci...368..742M. ("...initial exponential growth expected for an unconstrained outbreak.")
- ↑ Wiles, Siouxsie (9 March 2020). "The three phases of Covid-19—and how we can make it manageable". https://thespinoff.co.nz/society/09-03-2020/the-three-phases-of-covid-19-and-how-we-can-make-it-manageable/.
- ↑ 123.0 123.1 123.2 123.3 "How will country-based mitigation measures influence the course of the COVID-19 epidemic?". Lancet 395 (10228): 931–934. March 2020. doi:10.1016/S0140-6736(20)30567-5. PMID 32164834. "A key issue for epidemiologists is helping policy makers decide the main objectives of mitigation—e.g. minimising morbidity and associated mortality, avoiding an epidemic peak that overwhelms health-care services, keeping the effects on the economy within manageable levels, and flattening the epidemic curve to wait for vaccine development and manufacture on scale and antiviral drug therapies.".
- ↑ Barclay, Eliza (10 March 2020). "How canceled events and self-quarantines save lives, in one chart". https://www.vox.com/2020/3/10/21171481/coronavirus-us-cases-quarantine-cancellation.
- ↑ Barclay, Eliza; Scott, Dylan; Animashaun, Animashaun (7 April 2020). "The US doesn't just need to flatten the curve. It needs to "raise the line."". Vox. https://www.vox.com/2020/4/7/21201260/coronavirus-usa-chart-mask-shortage-ventilators-flatten-the-curve.
- ↑ 126.0 126.1 Wiles, Siouxsie (14 March 2020). "After 'Flatten the Curve', we must now 'Stop the Spread'. Here's what that means". https://thespinoff.co.nz/society/14-03-2020/after-flatten-the-curve-we-must-now-stop-the-spread-heres-what-that-means/.
- ↑ Grenfell, Rob; Drew, Trevor (17 February 2020). "Here's Why It's Taking So Long to Develop a Vaccine for the New Coronavirus". https://www.sciencealert.com/who-says-a-coronavirus-vaccine-is-18-months-away.
- ↑ 128.0 128.1 "COVID-19 Treatment Guidelines". National Institutes of Health. https://covid19treatmentguidelines.nih.gov/introduction/.
- ↑ 129.0 129.1 129.2 129.3 "Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review". JAMA. April 2020. doi:10.1001/jama.2020.6019. PMID 32282022.
- ↑ "Recommendation Regarding the Use of Cloth Face Coverings, Especially in Areas of Significant Community-Based Transmission". U.S. Centers for Disease Control and Prevention (CDC). 28 June 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover.html.
- ↑ 131.0 131.1 131.2 131.3 "Coronavirus Disease 2019 (COVID-19): Prevention & Treatment". 3 February 2020. https://www.cdc.gov/coronavirus/about/prevention.html.
- ↑ "Advice for Public". https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public.
- ↑ "My Hand-Washing Song: Readers Offer Lyrics For A 20-Second Scrub". https://www.npr.org/sections/goatsandsoda/2020/03/17/814221111/my-hand-washing-song-readers-offer-lyrics-for-a-20-second-scrub.
- ↑ "Wear masks in public says WHO, in update of COVID-19 advice". Reuters. 2020-06-05. https://www.reuters.com/article/us-health-coronavirus-who-masks-idUSKBN23C27Y.
- ↑ "When and how to use masks". https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks.
- ↑ 136.0 136.1 "Using face masks in the community—Technical Report". 8 April 2020. https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-use-face-masks-community.pdf.
- ↑ "Which countries have made wearing face masks compulsory?". Al Jazeera. 2020-05-20. https://www.aljazeera.com/news/2020/04/countries-wearing-face-masks-compulsory-200423094510867.html.
- ↑ "Recommendation Regarding the Use of Cloth Face Coverings, Especially in Areas of Significant Community-Based Transmission". 2020-02-11. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover.html.
- ↑ "For different groups of people: how to choose masks". NHC.gov.cn. National Health Commission of the People's Republic of China. 7 February 2020. http://en.nhc.gov.cn/2020-02/07/c_76337.htm. ""Disposable medical masks: Recommended for: · People in crowded places · Indoor working environment with a relatively dense population · People going to medical institutions · Children in kindergarten and students at school gathering to study and do other activities""
- ↑ "Face masks for the public during the covid-19 crisis". BMJ 369: m1435. April 2020. doi:10.1136/bmj.m1435. PMID 32273267. https://www.bmj.com/content/369/bmj.m1435.short.
- ↑ CDC (2020-02-11). "Caring for Someone Sick at Home". https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/care-for-someone.html.
- ↑ Maragakis, Lisa Lockerd. "Coronavirus, Social Distancing and Self Quarantine". Johns Hopkins University. https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/coronavirus-social-distancing-and-self-quarantine.
- ↑ Parker-Pope, Tara (19 March 2020). "Deciding How Much Distance You Should Keep". The New York Times. ISSN 0362-4331. https://www.nytimes.com/2020/03/19/well/live/coronavirus-quarantine-social-distancing.html.
- ↑ Systrom, Kevin; Krieger, Mike; O'Rourke, Ryan; Stein, Robby; Dellaert, Frank; Lerer, Adam (11 April 2020). "Rt Covid-19". https://rt.live/. Based on "Real time bayesian estimation of the epidemic potential of emerging infectious diseases". PLOS ONE 3 (5): e2185. May 2008. doi:10.1371/journal.pone.0002185. PMID 18478118. Bibcode: 2008PLoSO...3.2185B.
- ↑ "WHO-recommended handrub formulations". WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge Clean Care Is Safer Care.. World Health Organization. 19 March 2009. https://www.ncbi.nlm.nih.gov/books/NBK144054/. Retrieved 19 March 2020.
- ↑ "Coronavirus Disease 2019 (COVID-19)—Prevention & Treatment". U.S. Centers for Disease Control and Prevention (CDC). 10 March 2020. https://www.cdc.gov/coronavirus/2019-ncov/about/prevention.html.
- ↑ US EPA, OCSPP (13 March 2020). "List N: Disinfectants for Use Against SARS-CoV-2 (COVID-19)". https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2-covid-19.
- ↑ Lewis, Dyani (2020-07-08). "Mounting evidence suggests coronavirus is airborne — but health advice has not caught up" (in en). Nature. doi:10.1038/d41586-020-02058-1. https://www.nature.com/articles/d41586-020-02058-1.
- ↑ 149.0 149.1 149.2 149.3 Verbeek, Jos H.; Rajamaki, Blair; Ijaz, Sharea; Sauni, Riitta; Toomey, Elaine; Blackwood, Bronagh; Tikka, Christina; Ruotsalainen, Jani H. et al. (May 15, 2020). "Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff". The Cochrane Database of Systematic Reviews 5: CD011621. doi:10.1002/14651858.CD011621.pub5. ISSN 1469-493X. PMID 32412096. https://www.ncbi.nlm.nih.gov/pubmed/32412096.
- ↑ "Q&A: The novel coronavirus outbreak causing COVID-19". BMC Medicine 18 (1): 57. February 2020. doi:10.1186/s12916-020-01533-w. PMID 32106852.
- ↑ "Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province". Chinese Medical Journal 133 (9): 1025–1031. May 2020. doi:10.1097/CM9.0000000000000744. PMID 32044814.
- ↑ "Comorbidities and multi-organ injuries in the treatment of COVID-19". Lancet 395 (10228): e52. March 2020. doi:10.1016/s0140-6736(20)30558-4. PMID 32171074.
- ↑ "COVID-19, ECMO, and lymphopenia: a word of caution". The Lancet. Respiratory Medicine 8 (4): e24. April 2020. doi:10.1016/s2213-2600(20)30119-3. PMID 32178774.
- ↑ "Review of the 2019 novel coronavirus (SARS-CoV-2) based on current evidence". International Journal of Antimicrobial Agents 55 (6): 105948. March 2020. doi:10.1016/j.ijantimicag.2020.105948. PMID 32201353. PMC 7156162. https://www.sciencedirect.com/science/article/pii/S0924857920300984. Retrieved 27 March 2020.
- ↑ "Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures". Journal of Medical Virology n/a (n/a): 568–576. March 2020. doi:10.1002/jmv.25748. PMID 32134116.
- ↑ "2019 Novel coronavirus: where we are and what we know". Infection 48 (2): 155–163. April 2020. doi:10.1007/s15010-020-01401-y. PMID 32072569.
- ↑ "Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected". https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected.
- ↑ Farkas, Josh (March 2020) (digital). COVID-19—The Internet Book of Critical Care (Reference manual). USA: EMCrit. https://emcrit.org/ibcc/covid19/. Retrieved 13 March 2020.
- ↑ "COVID19—Resources for Health Care Professionals". Penn Libraries. 11 March 2020. https://guides.library.upenn.edu/covid-19.
- ↑ Roser, Max; Ritchie, Hannah; Ortiz-Ospina, Esteban (4 March 2020). "Coronavirus Disease (COVID-19)". Our World in Data. https://ourworldindata.org/coronavirus. Retrieved 12 March 2020.
- ↑ 161.0 161.1 161.2 "The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)—China, 2020". China CDC Weekly (Chinese Center for Disease Control and Prevention) 2 (8): 113–122. 17 February 2020. http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51. Retrieved 18 March 2020.
- ↑ (in ko) 코로나바이러스감염증-19 국내 발생 현황(7월 17일, 정례브리핑) (Report). Korea Centers for Disease Control and Prevention. 2020-07-17. https://www.cdc.go.kr/board/board.es?mid=a20501000000&bid=0015&list_no=367829&act=view. Retrieved 2020-07-17.
- ↑ (in Spanish) Actualización nº 109. Enfermedad por el coronavirus (COVID-19) (Report). Ministerio de Sanidad, Consumo y Bienestar Social. 18 May 2020. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Actualizacion_109_COVID-19.pdf. Retrieved 20 May 2020.
- ↑ "Epidemia COVID-19 – Bollettino sorveglianza integrata COVID-19" (in it). Istituto Superiore di Sanità. 5 June 2020. https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_3-giugno-2020.pdf.
- ↑ Roser, Max; Ritchie, Hannah; Ortiz-Ospina, Esteban (6 April 2020). "Coronavirus Disease (COVID-19)". Our World in Data. https://ourworldindata.org/coronavirus. Retrieved 6 April 2020.
- ↑ "Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents: A Systematic Review". JAMA Pediatrics. April 2020. doi:10.1001/jamapediatrics.2020.1467. PMID 32320004. https://jamanetwork.com/journals/jamapediatrics/fullarticle/2765169.
- ↑ "SARS-CoV-2 Infection in Children". New England Journal of Medicine (Massachusetts Medical Society) 382 (17): 1663–1665. April 2020. doi:10.1056/nejmc2005073. PMID 32187458.
- ↑ "Epidemiology of COVID-19 Among Children in China". Pediatrics 145 (6): e20200702. March 2020. doi:10.1542/peds.2020-0702. PMID 32179660. https://pediatrics.aappublications.org/content/pediatrics/early/2020/03/16/peds.2020-0702.full.pdf. Retrieved 16 March 2020.
- ↑ "Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?". The Lancet. Respiratory Medicine 8 (4): e21. April 2020. doi:10.1016/S0140-6736(20)30311-1. PMID 32171062.
- ↑ "Coronavirus Disease 2019 (COVID-19)". 11 February 2020. https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/children-faq.html.
- ↑ "COVID-19 and smoking: A systematic review of the evidence". Tobacco Induced Diseases 18 (March): 20. 20 March 2020. doi:10.18332/tid/119324. PMID 32206052.
- ↑ 172.0 172.1 172.2 Engin, Ayse Basak; Engin, Evren Doruk; Engin, Atilla (1 August 2020). "Two important controversial risk factors in SARS-CoV-2 infection: Obesity and smoking". Environmental Toxicology and Pharmacology 78: 103411. doi:10.1016/j.etap.2020.103411. ISSN 1382-6689. PMID 32422280. PMC 7227557. https://www.sciencedirect.com/science/article/pii/S1382668920300879. Retrieved 31 May 2020.
- ↑ Tamara, Alice; Tahapary, Dicky L. (1 July 2020). "Obesity as a predictor for a poor prognosis of COVID-19: A systematic review". Diabetes & Metabolic Syndrome: Clinical Research & Reviews 14 (4): 655–659. doi:10.1016/j.dsx.2020.05.020. ISSN 1871-4021. PMID 32438328. PMC 7217103. https://www.sciencedirect.com/science/article/pii/S1871402120301399. Retrieved 31 May 2020.
- ↑ "Obesity ‑ a risk factor for increased COVID‑19 prevalence, severity and lethality (Review)". Molecular Medicine Reports. https://www.researchgate.net/publication/341162753. Retrieved 31 May 2020.
- ↑ John Parkinson, “Study: Majority of Children with COVID-19 Have Mild Disease, Mortality is Rare”, Contagion Live, 25 June 2020.
- ↑ "WHO Director-General's statement on the advice of the IHR Emergency Committee on Novel Coronavirus". https://www.who.int/dg/speeches/detail/who-director-general-s-statement-on-the-advice-of-the-ihr-emergency-committee-on-novel-coronavirus.
- ↑ 177.0 177.1 Characteristics of COVID-19 patients dying in Italy Report based on available data on April 2nd, 2020 (Report). Istituto Superiore di Sanità. 3 April 2020. https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_2_april_2020.pdf. Retrieved 3 April 2020.
- ↑ "Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China". Journal of Medical Virology 92 (4): 441–447. April 2020. doi:10.1002/jmv.25689. PMID 31994742.
- ↑ "Coronavirus Age, Sex, Demographics (COVID-19)". https://www.worldometers.info/coronavirus/coronavirus-age-sex-demographics/.
- ↑ "Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019—COVID-NET, 14 States, 1–30 March 2020". MMWR. Morbidity and Mortality Weekly Report 69 (15): 458–464. April 2020. doi:10.15585/mmwr.mm6915e3. PMID 32298251. https://www.cdc.gov/mmwr/volumes/69/wr/mm6915e3.htm.
- ↑ CDC (2020-02-11). "Coronavirus Disease 2019 (COVID-19)" (in en-us). https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html.
- ↑ Zhao, Qianwen; Meng, Meng; Kumar, Rahul; Wu, Yinlian; Huang, Jiaofeng; Lian, Ningfang; Deng, Yunlei; Lin, Su (2020-05-17). "The impact of COPD and smoking history on the severity of COVID‐19: A systemic review and meta‐analysis". Journal of Medical Virology. doi:10.1002/jmv.25889. ISSN 0146-6615. http://dx.doi.org/10.1002/jmv.25889.
- ↑ "Smoking and COVID-19" (in en). https://www.who.int/news-room/commentaries/detail/smoking-and-covid-19.
- ↑ "Coronavirus Disease 2019 (COVID-19)". 11 February 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html.
- ↑ DeRobertis, Jacqueline (3 May 2020). "People who use drugs are more vulnerable to coronavirus. Here's what clinics are doing to help.". https://www.theadvocate.com/baton_rouge/news/coronavirus/article_f80cf77e-84fa-11ea-88d5-2b37dc9dd966.html.
- ↑ "Features, Evaluation and Treatment Coronavirus (COVID-19)". StatPearls. Treasure Island (FL): StatPearls Publishing. 2020. https://www.ncbi.nlm.nih.gov/books/NBK554776/. Retrieved 18 March 2020.
- ↑ "COVID-19: what is next for public health?". Lancet 395 (10224): 542–545. February 2020. doi:10.1016/s0140-6736(20)30374-3. PMID 32061313.
- ↑ "Cardiovascular complications in COVID-19". The American Journal of Emergency Medicine 38 (7): 1504–1507. April 2020. doi:10.1016/j.ajem.2020.04.048. PMID 32317203.
- ↑ "Liver injury during highly pathogenic human coronavirus infections". Liver International 40 (5): 998–1004. May 2020. doi:10.1111/liv.14435. PMID 32170806.
- ↑ "Neurological complications of coronavirus and COVID-19". Revista de Neurología 70 (9): 311–322. May 2020. doi:10.33588/rn.7009.2020179. PMID 32329044.
- ↑ "Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19". https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. Retrieved 20 May 2020.
- ↑ HAN Archive—00432 (Report). 15 May 2020. https://emergency.cdc.gov/han/2020/han00432.asp. Retrieved 20 May 2020.
- ↑ Cheung, Elizabeth (13 March 2020). "Some recovered Covid-19 patients may have lung damage, doctors say". https://www.scmp.com/news/hong-kong/health-environment/article/3074988/coronavirus-some-recovered-patients-may-have.
- ↑ Servick, Kelly (8 April 2020). "For survivors of severe COVID-19, beating the virus is just the beginning". Science. doi:10.1126/science.abc1486. ISSN 0036-8075. https://www.sciencemag.org/news/2020/04/survivors-severe-covid-19-beating-virus-just-beginning.
- ↑ "BSI open letter to Government on SARS-CoV-2 outbreak response". British Society for Immunology. https://www.immunology.org/news/bsi-open-letter-government-sars-cov-2-outbreak-response.
- ↑ Schraer, Rachel (25 April 2020). "Coronavirus: Immunity passports 'could increase virus spread'". https://www.bbc.com/news/world-52425825.
- ↑ "Can you get coronavirus twice or does it cause immunity?". 13 March 2020. https://www.independent.co.uk/life-style/health-and-families/coronavirus-immunity-reinfection-get-covid-19-twice-sick-spread-relapse-a9400691.html.
- ↑ Politi, Daniel (11 April 2020). "WHO Investigating Reports of Coronavirus Patients Testing Positive Again After Recovery". Slate. https://slate.com/news-and-politics/2020/04/who-reports-coronavirus-testing-positive-recovery.html.
- ↑ "They survived the coronavirus. Then they tested positive again. Why?". 13 March 2020. https://www.latimes.com/world-nation/story/2020-03-13/china-japan-korea-coronavirus-reinfection-test-positive.
- ↑ "14% of Recovered Covid-19 Patients in Guangdong Tested Positive Again". Caixin Global. https://www.caixinglobal.com/2020-02-26/14-of-recovered-covid-19-patients-in-guangdong-tested-positive-again-101520415.html.
- ↑ 201.0 201.1 "The COVID-19 Pandemic in the US: A Clinical Update". JAMA. April 2020. doi:10.1001/jama.2020.5788. PMID 32250388.
- ↑ Parry, Richard Lloyd (30 April 2020), "Coronavirus patients can't relapse, South Korean scientists believe", The Times, https://www.thetimes.co.uk/article/coronavirus-patients-cant-relapse-south-korean-scientists-believe-rkm8zm7d9
- ↑ "Findings from investigation and analysis of re-positive cases" (in en). 19 May 2020. https://www.cdc.go.kr/board/board.es?mid=a30402000000&bid=0030.
- ↑ "What if immunity to covid-19 doesn't last?". https://www.technologyreview.com/2020/04/27/1000569/how-long-are-people-immune-to-covid-19/.
- ↑ "Direct observation of repeated infections with endemic coronaviruses". Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University. 15 April 2020. http://www.columbia.edu/~jls106/galanti_shaman_ms_supp.pdf.
- ↑ Berger, Kevin (12 March 2020). "The Man Who Saw the Pandemic Coming". https://nautil.us/issue/83/intelligence/the-man-who-saw-the-pandemic-coming.
- ↑ "The outbreak of COVID-19: An overview". Journal of the Chinese Medical Association 83 (3): 217–220. March 2020. doi:10.1097/JCMA.0000000000000270. PMID 32134861.
- ↑ "A novel coronavirus outbreak of global health concern". Lancet 395 (10223): 470–473. February 2020. doi:10.1016/S0140-6736(20)30185-9. PMID 31986257. PMC 7135038. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30185-9/abstract.
- ↑ Cohen, Jon (January 2020). "Wuhan seafood market may not be source of novel virus spreading globally". Science. doi:10.1126/science.abb0611. https://www.sciencemag.org/news/2020/01/wuhan-seafood-market-may-not-be-source-novel-virus-spreading-globally.
- ↑ "Novel Coronavirus—China". 12 January 2020. https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/.
- ↑ Kessler, Glenn (17 April 2020). "Trump's false claim that the WHO said the coronavirus was 'not communicable'". The Washington Post. https://www.washingtonpost.com/politics/2020/04/17/trumps-false-claim-that-who-said-coronavirus-was-not-communicable/.
- ↑ Kuo, Lily (21 January 2020). "China confirms human-to-human transmission of coronavirus". The Guardian. https://www.theguardian.com/world/2020/jan/20/coronavirus-spreads-to-beijing-as-china-confirms-new-cases.
- ↑ Novel Coronavirus Pneumonia Emergency Response Epidemiology Team (February 2020). "[The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]" (in zh). Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi 41 (2): 145–151. doi:10.3760/cma.j.issn.0254-6450.2020.02.003. PMID 32064853.
- ↑ Areddy, James T. (26 May 2020). "China Rules Out Animal Market and Lab as Coronavirus Origin". The Wall Street Journal. https://www.wsj.com/articles/china-rules-out-animal-market-and-lab-as-coronavirus-origin-11590517508. Retrieved 29 May 2020.
- ↑ Kelland, Kate (19 June 2020). "Italy sewage study suggests COVID-19 was there in December 2019". Reuters. https://www.reuters.com/article/us-health-coronavirus-italy-sewage/italy-sewage-study-suggests-covid-19-was-there-in-december-2019-idUSKBN23Q1J9.
- ↑ Duarte, Fernando (24 February 2020). "As the cases of coronavirus increase in China and around the world, the hunt is on to identify "patient zero".". BBC News Online. https://www.bbc.com/future/article/20200221-coronavirus-the-harmful-hunt-for-covid-19s-patient-zero.
- ↑ "COVID-19: what is next for public health?". Lancet 395 (10224): 542–545. February 2020. doi:10.1016/S0140-6736(20)30374-3. PMID 32061313.
- ↑ March 2020, Jeanna Bryner-Live Science Editor-in-Chief 14. "1st known case of coronavirus traced back to November in China". https://www.livescience.com/first-case-coronavirus-found.html.
- ↑ Politics, Canadian (8 April 2020). "The birth of a pandemic: How COVID-19 went from Wuhan to Toronto | National Post". https://nationalpost.com/news/politics/the-birth-of-a-pandemic-how-covid-19-went-from-wuhan-to-toronto.
- ↑ 高昱 (26 February 2020). "独家 | 新冠病毒基因测序溯源:警报是何时拉响的" (in zh). Caixin. http://china.caixin.com/2020-02-26/101520972.html.
- ↑ "最早上报疫情的她,怎样发现这种不一样的肺炎" (in zh-cn). 北京. https://news.china.com/zw/news/13000776/20200209/37780703.html. Retrieved 11 February 2020.
- ↑ "Undiagnosed pneumonia—China (HU): RFI". ProMED. https://promedmail.org/promed-post/?id=6864153. Retrieved 7 May 2020.
- ↑ "'Hero who told the truth': Chinese rage over coronavirus death of whistleblower doctor". The Guardian. 7 February 2020. https://www.theguardian.com/global-development/2020/feb/07/coronavirus-chinese-rage-death-whistleblower-doctor-li-wenliang.
- ↑ Kuo, Lily (11 March 2020). "Coronavirus: Wuhan doctor speaks out against authorities". The Guardian (London). https://www.theguardian.com/world/2020/mar/11/coronavirus-wuhan-doctor-ai-fen-speaks-out-against-authorities.
- ↑ "Novel Coronavirus". World Health Organization (WHO). https://www.who.int/westernpacific/emergencies/novel-coronavirus.
- ↑ "武汉现不明原因肺炎 官方确认属实:已经做好隔离". Xinhua Net 新華網. 31 December 2019. https://news.163.com/19/1231/10/F1NGTJNJ00019K82.html.
- ↑ "Archived copy" (in zh). WJW.Wuhan.gov.cn. Wuhan Municipal Health Commission. 31 December 2019. http://wjw.wuhan.gov.cn/front/web/showDetail/2019123108989.
- ↑ "Mystery pneumonia virus probed in China". BBC News Online. 3 January 2020. https://www.bbc.com/news/world-asia-china-50984025.
- ↑ "Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia". New England Journal of Medicine 382 (13): 1199–1207. March 2020. doi:10.1056/NEJMoa2001316. PMID 31995857.
- ↑ WHO–China Joint Mission (16–24 February 2020). "Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19)". World Health Organization. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf.
- ↑ "China confirms sharp rise in cases of SARS-like virus across the country". 20 January 2020. https://www.france24.com/en/20200120-china-confirms-sharp-rise-in-cases-of-sars-like-virus-across-the-country.
- ↑ The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team (17 February 2020). "The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)—China, 2020". China CDC Weekly 2 (8): 113–122. http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51. Retrieved 18 March 2020.
- ↑ 233.0 233.1 "Flattery and foot dragging: China's influence over the WHO under scrutiny". The Globe and Mail. 25 April 2020. https://www.theglobeandmail.com/world/article-flattery-and-foot-dragging-chinas-influence-over-the-who-under/.
- ↑ "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China". Lancet 395 (10223): 497–506. 24 January 2020. doi:10.1016/S0140-6736(20)30183-5. PMID 31986264. PMC 7159299. https://www.thelancet.com/action/showPdf?pii=S0140-6736%2820%2930183-5.
- ↑ Horton, Richard (18 March 2020). "Scientists have been sounding the alarm on coronavirus for months. Why did Britain fail to act?". http://www.theguardian.com/commentisfree/2020/mar/18/coronavirus-uk-expert-advice-wrong.
- ↑ "China delayed releasing coronavirus info, frustrating WHO". 2 June 2020. https://apnews.com/3c061794970661042b18d5aeaaed9fae.
- ↑ "Coronavirus: Primi due casi in Italia" (in it). Corriere della sera. 31 January 2020. https://www.corriere.it/cronache/20_gennaio_30/coronavirus-italia-corona-9d6dc436-4343-11ea-bdc8-faf1f56f19b7.shtml.
- ↑ Fredericks, Bob (13 March 2020). "WHO says Europe is new epicenter of coronavirus pandemic". New York Post. https://nypost.com/2020/03/13/who-says-europe-is-new-epicenter-of-coronavirus-pandemic/. Retrieved 9 May 2020.
- ↑ "Coronavirus: Number of COVID-19 deaths in Italy surpasses China as total reaches 3,405". Sky News. https://news.sky.com/story/coronavirus-number-of-covid-19-deaths-in-italy-surpasses-china-as-total-reaches-3-405-11960412. Retrieved 7 May 2020.
- ↑ McNeil Jr., Donald G. (26 March 2020). "The U.S. Now Leads the World in Confirmed Coronavirus Cases". The New York Times. https://www.nytimes.com/2020/03/26/health/usa-coronavirus-cases.html.
- ↑ "Studies Show N.Y. Outbreak Originated in Europe". The New York Times. 8 April 2020. https://www.nytimes.com/2020/04/08/us/coronavirus-live-updates.html.
- ↑ Irish, John (4 May 2020). "After retesting samples, French hospital discovers COVID-19 case from December". Reuters. https://www.reuters.com/article/us-health-coronavirus-france-idUSKBN22G20L.
- ↑ "SARS-COV-2 was already spreading in France in late December 2019". International Journal of Antimicrobial Agents 55 (6): 106006. 3 May 2020. doi:10.1016/j.ijantimicag.2020.106006. PMID 32371096.
- ↑ "2 died with coronavirus weeks before 1st U.S. virus death". 22 April 2020. https://www.pbs.org/newshour/nation/2-died-with-coronavirus-weeks-before-1st-u-s-virus-death.
- ↑ 245.0 245.1 "Beijing Covid-19 outbreak puts food markets back in infection focus". 16 June 2020. https://www.scmp.com/news/china/society/article/3089161/beijing-covid-19-outbreak-puts-food-markets-back-infection-focus.
- ↑ "北京连续确诊3例新冠患者 新发地批发市场暂停营业". http://www.caixin.com/2020-06-12/101566522.html.
- ↑ Gan, Nectar. "China's new coronavirus outbreak sees Beijing adopt 'wartime' measures". https://www.cnn.com/2020/06/15/asia/coronavirus-beijing-outbreak-intl-hnk/index.html.
- ↑ "Beijing logs record 36 COVID-19 cases, linked to market cluster". https://www.channelnewsasia.com/news/asia/covid-19-cases-china-beijing-market-cluster-12833874.
- ↑ "Principles of Epidemiology | Lesson 3—Section 3". 18 February 2019. https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section3.html.
- ↑ Ritchie, Hannah; Roser, Max (25 March 2020). "What do we know about the risk of dying from COVID-19?". in Chivers, Tom. https://ourworldindata.org/covid-mortality-risk.
- ↑ "COVID-19 in Italy: momentous decisions and many uncertainties". The Lancet. Global Health 8 (5): e641–e642. May 2020. doi:10.1016/S2214-109X(20)30110-8. PMID 32199072. PMC 7104294. https://www.thelancet.com/journals/langlo/article/PIIS2214-109X(20)30110-8/abstract.
- ↑ "What do we know about the risk of dying from COVID-19?". https://ourworldindata.org/covid-mortality-risk.
- ↑ 253.0 253.1 "COVID-19 in Prisons and Jails in the United States". JAMA Internal Medicine. April 2020. doi:10.1001/jamainternmed.2020.1856. PMID 32343355.
- ↑ Waldstein, David (6 May 2020). "To Fight Virus in Prisons, C.D.C. Suggests More Screenings". https://www.nytimes.com/2020/05/06/health/coronavirus-prisons-cdc.html.
- ↑ "Total confirmed cases of COVID-19 per million people". https://ourworldindata.org/grapher/total-confirmed-cases-of-covid-19-per-million-people.
- ↑ "Total confirmed deaths due to COVID-19 per million people". https://ourworldindata.org/grapher/total-covid-deaths-per-million.
- ↑ "What do we know about the risk of dying from COVID-19?". https://ourworldindata.org/covid-mortality-risk.
- ↑ "Coronavirus disease 2019 (COVID-19) Situation Report—30". 19 February 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200219-sitrep-30-covid-19.pdf.
- ↑ "Coronavirus disease 2019 (COVID-19) Situation Report—31". 20 February 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200220-sitrep-31-covid-19.pdf.
- ↑ McNeil Jr., Donald G. (4 July 2020). "The Pandemic’s Big Mystery: How Deadly Is the Coronavirus? – Even with more than 500,000 dead worldwide, scientists are struggling to learn how often the virus kills. Here’s why.". The New York Times. https://www.nytimes.com/2020/07/04/health/coronavirus-death-rate.html. Retrieved 6 July 2020.
- ↑ "Global Research and Innovation Forum on COVID-19: Virtual Press Conference". World Health Organization. July 2, 2020. https://www.who.int/docs/default-source/coronaviruse/virtual-press-conference---2-july---update-on-covid-19-r-d.pdf.
- ↑ "Coronavirus Disease 2019 (COVID-19)". 11 February 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html. Retrieved 22 May 2020.
- ↑ Gan, Nectar; McKeehan, Brett (2020-07-11). "CDC now estimates that 40% of people infected with Covid-19 don't have any symptoms" (in en). https://edition.cnn.com/world/live-news/coronavirus-pandemic-07-11-20-intl/h_d6a63735c82f708ffdcff33082da393b.
- ↑ "Global Covid-19 Case Fatality Rates". 17 March 2020. https://www.cebm.net/covid-19/global-covid-19-case-fatality-rates/.
- ↑ Haake, Daniel (24 April 2020). "Gangelt–A representative study on the lethality of COVID-19". https://towardsdatascience.com/gangelt-a-representative-study-on-the-lethality-of-covid-19-5d877dbd6e55.
- ↑ Vogel, Gretchen (21 April 2020). "Antibody surveys suggesting vast undercount of coronavirus infections may be unreliable". https://www.sciencemag.org/news/2020/04/antibody-surveys-suggesting-vast-undercount-coronavirus-infections-may-be-unreliable.
- ↑ "The Coronavirus Isn't Just the Flu, Bro". https://www.bloomberg.com/opinion/articles/2020-04-24/is-coronavirus-worse-than-the-flu-blood-studies-say-yes-by-far.
- ↑ Mole, Beth (24 April 2020). "Experts demolish studies suggesting COVID-19 is no worse than flu". https://arstechnica.com/science/2020/04/experts-demolish-studies-suggesting-covid-19-is-no-worse-than-flu/.
- ↑ Wilson, Linus (May 2020). "SARS-CoV-2, COVID-19, Infection Fatality Rate (IFR) Implied by the Serology, Antibody, Testing in New York City". SSRN 3590771.
- ↑ "COVID-19: Data". City of New York. https://www1.nyc.gov/site/doh/covid/covid-19-data.page.
- ↑ Modi, Chirag (21 April 2020). "How deadly is COVID-19? Data Science offers answers from Italy mortality data.". https://medium.com/bccp-uc-berkeley/how-deadly-is-covid-19-data-science-offers-answers-from-italy-mortality-data-58abedf824cf.
- ↑ "COVID-19: the gendered impacts of the outbreak". Lancet 395 (10227): 846–848. March 2020. doi:10.1016/S0140-6736(20)30526-2. PMID 32151325.
- ↑ Novel Coronavirus Pneumonia Emergency Response Epidemiology Team (February 2020). "[The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China"] (in ch). Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi 41 (2): 145–151. doi:10.3760/cma.j.issn.0254-6450.2020.02.003. PMID 32064853. http://rs.yiigle.com/yufabiao/1181998.htm.
- ↑ "The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19)". China CDC Weekly 2 (8): 113–122. 1 February 2020. doi:10.46234/ccdcw2020.032. ISSN 2096-7071. http://weekly.chinacdc.cn/en/article/id/e53946e2-c6c4-41e9-9a9b-fea8db1a8f51. Retrieved 15 June 2020.
- ↑ Hu, Yong; Sun, Jiazhong; Dai, Zhe; Deng, Haohua; Li, Xin; Huang, Qi; Wu, Yuwen; Sun, Li et al. (1 June 2020). "Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis". Journal of Clinical Virology 127: 104371. doi:10.1016/j.jcv.2020.104371. ISSN 1386-6532. PMID 32315817. PMC 7195434. https://www.sciencedirect.com/science/article/pii/S138665322030113X. Retrieved 15 June 2020.
- ↑ Fu, Leiwen; Wang, Bingyi; Yuan, Tanwei; Chen, Xiaoting (1 June 2020). "Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis". Journal of Infection 80 (6): 656–665. doi:10.1016/j.jinf.2020.03.041. ISSN 0163-4453. PMID 32283155. PMC 7151416. https://www.sciencedirect.com/science/article/pii/S0163445320301705. Retrieved 15 June 2020.
- ↑ Yuki, Koichi; Fujiogi, Miho; Koutsogiannaki, Sophia (1 June 2020). "COVID-19 pathophysiology: A review". Clinical Immunology 215: 108427. doi:10.1016/j.clim.2020.108427. ISSN 1521-6616. PMID 32325252. PMC 7169933. https://www.sciencedirect.com/science/article/pii/S152166162030262X#bb0125. Retrieved 15 June 2020.
- ↑ Rabin, Roni Caryn (20 March 2020). "In Italy, Coronavirus Takes a Higher Toll on Men". The New York Times. https://www.nytimes.com/2020/03/20/health/coronavirus-italy-men-risk.html. Retrieved 7 April 2020.
- ↑ "COVID-19 weekly surveillance report". http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/weekly-surveillance-report. Retrieved 7 April 2020.
- ↑ 280.0 280.1 Gupta, Alisha Haridasani (3 April 2020). "Does Covid-19 Hit Women and Men Differently? U.S. Isn't Keeping Track". The New York Times. https://www.nytimes.com/2020/04/03/us/coronavirus-male-female-data-bias.html. Retrieved 7 April 2020.
- ↑ Salje, Henrik; Tran Kiem, Cécile; Lefrancq, Noémie; Courtejoie, Noémie; Bosetti, Paolo; Paireau, Juliette; Andronico, Alessio; Hozé, Nathanaël et al. (13 May 2020). "Estimating the burden of SARS-CoV-2 in France" (in en). Science: Table S1 and S2 in Supplementary Materials. doi:10.1126/science.abc3517. ISSN 0036-8075. PMID 32404476.
- ↑ 282.0 282.1 "COVID-19 exacerbating inequalities in the US". Lancet 395 (10232): 1243–1244. April 2020. doi:10.1016/S0140-6736(20)30893-X. PMID 32305087.
- ↑ "Population-Based Estimates of Chronic Conditions Affecting Risk for Complications from Coronavirus Disease, United States". Emerging Infectious Diseases 26 (8). April 2020. doi:10.3201/eid2608.200679. PMID 32324118.
- ↑ "COVID-19 Presents Significant Risks for American Indian and Alaska Native People". 14 May 2020. https://www.kff.org/coronavirus-covid-19/issue-brief/covid-19-presents-significant-risks-for-american-indian-and-alaska-native-people/.
- ↑ "COVID-19 Presents Significant Risks for American Indian and Alaska Native People". 14 May 2020. https://www.kff.org/coronavirus-covid-19/issue-brief/covid-19-presents-significant-risks-for-american-indian-and-alaska-native-people/.
- ↑ "The COVID-19 Pandemic: a Call to Action to Identify and Address Racial and Ethnic Disparities". Journal of Racial and Ethnic Health Disparities 7 (3): 398–402. April 2020. doi:10.1007/s40615-020-00756-0. PMID 32306369.
- ↑ "How coronavirus deaths in the UK compare by race and ethnicity". 9 June 2020. https://www.independent.co.uk/news/uk/home-news/coronavirus-death-toll-uk-race-white-black-asian-bame-ethnicity-cases-a9557076.html.
- ↑ "Emerging findings on the impact of COVID-19 on black and minority ethnic people". https://www.health.org.uk/news-and-comment/charts-and-infographics/emerging-findings-on-the-impact-of-covid-19-on-black-and-min.
- ↑ Butcher, Benjamin; Massey, Joel (9 June 2020). "Why are more BAME people dying from coronavirus?". BBC News Online. https://www.bbc.com/news/uk-52219070.
- ↑ "2nd U.S. Case Of Wuhan Coronavirus Confirmed". https://www.npr.org/sections/health-shots/2020/01/24/799208865/a-second-u-s-case-of-wuhan-coronavirus-is-confirmed.
- ↑ McNeil Jr, Donald G. (2 February 2020). "Wuhan Coronavirus Looks Increasingly Like a Pandemic, Experts Say". The New York Times. ISSN 0362-4331. https://www.nytimes.com/2020/02/02/health/coronavirus-pandemic-china.html.
- ↑ Griffiths, James. "Wuhan coronavirus deaths spike again as outbreak shows no signs of slowing". https://www.cnn.com/2020/02/05/asia/wuhan-coronavirus-update-death-toll-spike-intl-hnk/index.html.
- ↑ "A novel coronavirus (2019-nCoV) causing pneumonia-associated respiratory syndrome". Cellular & Molecular Immunology 17 (5): 554. May 2020. doi:10.1038/s41423-020-0372-4. PMID 32024976.
- ↑ "A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster". Lancet 395 (10223): 514–523. February 2020. doi:10.1016/S0140-6736(20)30154-9. PMID 31986261.
- ↑ Shablovsky, Suzanne (22 September 2017). "The legacy of the Spanish flu". Science 357 (6357): 1245. doi:10.1126/science.aao4093. ISSN 0036-8075. Bibcode: 2017Sci...357.1245S. https://science.sciencemag.org/content/357/6357/1245.
- ↑ "Stop the coronavirus stigma now". Nature: pp. 165. 7 April 2020. doi:10.1038/d41586-020-01009-0. https://www.nature.com/articles/d41586-020-01009-0.
- ↑ "Novel Coronavirus (2019-nCoV) SITUATION REPORT—1". 21 January 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf.
- ↑ "Novel Coronavirus(2019-nCoV) Situation Report—10". 30 January 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200130-sitrep-10-ncov.pdf.
- ↑ "Novel coronavirus named 'Covid-19': WHO". TODAYonline. https://www.todayonline.com/world/wuhan-novel-coronavirus-named-covid-19-who.
- ↑ "The coronavirus spreads racism against—and among—ethnic Chinese". The Economist. 17 February 2020. https://www.economist.com/china/2020/02/17/the-coronavirus-spreads-racism-against-and-among-ethnic-chinese.
- ↑ "World Health Organization Best Practices for the Naming of New Human Infectious Diseases". May 2015. https://apps.who.int/iris/bitstream/handle/10665/163636/WHO_HSE_FOS_15.1_eng.pdf.
- ↑ 302.0 302.1 "Naming the coronavirus disease (COVID-19) and the virus that causes it". https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it.
- ↑ Coronavirus disease 2019 (COVID-19) in the EU/EEA and the UK—eighth update (Report). ecdc. https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-rapid-risk-assessment-coronavirus-disease-2019-eighth-update-8-april-2020.pdf. Retrieved 19 April 2020.
- ↑ "China coronavirus: Misinformation spreads online about origin and scale". BBC News Online. 30 January 2020. https://www.bbc.com/news/blogs-trending-51271037.
- ↑ Taylor, Josh (31 January 2020). "Bat soup, dodgy cures and 'diseasology': the spread of coronavirus misinformation". The Guardian. https://www.theguardian.com/world/2020/jan/31/bat-soup-dodgy-cures-and-diseasology-the-spread-of-coronavirus-bunkum.
- ↑ "Here's A Running List Of Disinformation Spreading About The Coronavirus". https://www.buzzfeednews.com/article/janelytvynenko/coronavirus-disinformation-spread.
- ↑ "Reduction in ST-Segment Elevation Cardiac Catheterization Laboratory Activations in the United States during COVID-19 Pandemic". Journal of the American College of Cardiology. April 2020. doi:10.1016/j.jacc.2020.04.011. PMID 32283124.
- ↑ 308.0 308.1 'Where are all our patients?': Covid phobia is keeping people with serious heart symptoms away from ERs, Stat News, Usha Lee McFarling, 23 April 2020.
- ↑ Faust, Jeremy Samuel (28 April 2020). "Medication Shortages Are the Next Crisis". The Atlantic. https://www.theatlantic.com/ideas/archive/2020/04/medication-shortages-are-next-crisis/610798/. Retrieved 17 May 2020.
- ↑ "Sexually transmitted infections surveillance reports – Reports". https://www.health.nsw.gov.au/Infectious/reports/Pages/STI-reports.aspx.
- ↑ Wareham, Jamie. "U.K. Lockdown Has 'Broken HIV Chain' With Huge Reduction In New STI Cases". https://www.forbes.com/sites/jamiewareham/2020/05/01/uk-lockdown-has-broken-hiv-chain-with-huge-reduction-in-new-sti-cases/.
- ↑ Cowling, Benjamin J.; Ali, Sheikh Taslim; Ng, Tiffany W. Y.; Tsang, Tim K.; Li, Julian C. M.; Fong, Min Whui; Liao, Qiuyan; Kwan, Mike YW et al. (1 May 2020). "Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study". The Lancet Public Health 5 (5): e279–e288. doi:10.1016/S2468-2667(20)30090-6. ISSN 2468-2667. PMID 32311320. PMC 7164922. https://www.thelancet.com/journals/lanpub/article/PIIS2468-2667(20)30090-6/abstract.
- ↑ Klein, Alice. "Australia sees huge decrease in flu cases due to coronavirus measures". https://www.newscientist.com/article/2242113-australia-sees-huge-decrease-in-flu-cases-due-to-coronavirus-measures/.
- ↑ "Weekly U.S. Influenza Surveillance Report (FluView)". 8 May 2020. https://www.cdc.gov/flu/weekly/index.htm.
- ↑ "The psychological impact of quarantine and how to reduce it: rapid review of the evidence" (in en). The Lancet 395 (10227): 912–920. 14 March 2020. doi:10.1016/S0140-6736(20)30460-8. ISSN 0140-6736. PMID 32112714. PMC 7158942. https://www.thelancet.com/action/showPdf?pii=S0140-6736%2820%2930460-8. Retrieved 20 March 2020.
- ↑ "Coronavirus: Belgian cat infected by owner". Brusselstimes.com. 27 March 2020. https://www.brusselstimes.com/all-news/belgium-all-news/103003/coronavirus-belgian-woman-infected-her-cat/.
- ↑ Goldstein, Joseph (6 April 2020). "Bronx Zoo Tiger Is Sick With the Coronavirus". https://www.nytimes.com/2020/04/06/nyregion/bronx-zoo-tiger-coronavirus.html.
- ↑ "Coronavirus hits Netherlands farm animals as minks test positive for virus". Fox News. 26 April 2020. https://www.foxnews.com/world/mink-netherlands-coronavirus-farm.
- ↑ "Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2". Science 368 (6494): 1016–1020. April 2020. doi:10.1126/science.abb7015. PMID 32269068.
- ↑ "Simulation of the clinical and pathological manifestations of Coronavirus Disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility". Clinical Infectious Diseases. March 2020. doi:10.1093/cid/ciaa325. ISSN 1058-4838. PMID 32215622. PMC 7184405. https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciaa325/32967819/ciaa325.pdf.
- ↑ 321.0 321.1 321.2 "Therapeutic options for the 2019 novel coronavirus (2019-nCoV)". Nature Reviews. Drug Discovery 19 (3): 149–150. March 2020. doi:10.1038/d41573-020-00016-0. PMID 32127666.
- ↑ "COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics". Human Vaccines & Immunotherapeutics 16 (6): 1232–1238. March 2020. doi:10.1080/21645515.2020.1735227. PMID 32186952.
- ↑ "Potential interventions for novel coronavirus in China: A systematic review". Journal of Medical Virology 92 (5): 479–490. May 2020. doi:10.1002/jmv.25707. PMID 32052466.
- ↑ 324.0 324.1 Kupferschmidt, Kai; Cohen, Jon (22 March 2020). "WHO launches global megatrial of the four most promising coronavirus treatments". Science Magazine. doi:10.1126/science.abb8497. https://www.sciencemag.org/news/2020/03/who-launches-global-megatrial-four-most-promising-coronavirus-treatments. Retrieved 27 March 2020.
- ↑ "Citing safety concerns, the W.H.O. paused tests of a drug Trump said he had taken.". The New York Times. 26 May 2020. https://www.nytimes.com/2020/05/26/world/coronavirus-news.html.
- ↑ "France bans use of hydroxychloroquine, drug touted by Trump, in coronavirus patients". CBS News. 27 May 2020. https://www.cbsnews.com/news/france-bans-use-of-hydroxychloroquine-drug-touted-by-trump-to-treat-coronavirus/.
- ↑ Bradley-Ridout, Glyneva; Fuller, Kaitlin; Gray, Mikaela; Nekolaichuk, Erica (9 April 2020). "Navigating the COVID-19 Evidence Landscape". University of Toronto Libraries—Gerstein Science Information Centre. http://www.covidliterature.ca/.
- ↑ "Features, Evaluation and Treatment Coronavirus (COVID-19)". StatPearls [Internet]. StatPearls. March 2020. Bookshelf ID: NBK554776. https://www.ncbi.nlm.nih.gov/books/NBK554776/.
- ↑ 329.0 329.1 329.2 WHO team. "Draft Landscape of COVID-19 Candidate Vaccines." World Health Organization, World Health Organization, 29 June 2020, www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
- ↑ 330.0 330.1 Vaccitech. "Technology." Vaccitech, 21 May 2020, www.vaccitech.co.uk/technology.
- ↑ "An Open Study of the Safety, Tolerability and Immunogenicity of the Drug 'Gam-COVID-Vac' Vaccine Against COVID-19 – Full Text View." Full Text View – ClinicalTrials.gov, 22 June 2020, clinicaltrials.gov/ct2/show/NCT04436471?term=vaccine.
- ↑ "The SARS-CoV-2 Vaccine Pipeline: an Overview". Current Tropical Medicine Reports 7 (2): 61–64. March 2020. doi:10.1007/s40475-020-00201-6. PMID 32219057.
- ↑ "News Feature: Avoiding pitfalls in the pursuit of a COVID-19 vaccine". Proceedings of the National Academy of Sciences of the United States of America (Proceedings of the National Academy of Sciences) 117 (15): 8218–8221. April 2020. doi:10.1073/pnas.2005456117. PMID 32229574.
- ↑ 334.0 334.1 334.2 "COVID-19 treatment and vaccine tracker". Milken Institute. 21 April 2020. https://milkeninstitute.org/sites/default/files/2020-04/Covid19%20Tracker%20NEW4-21-20-2.pdf.
- ↑ 335.0 335.1 335.2 335.3 Koch, Selina; Pong, Winnie (13 March 2020). "First up for COVID-19: nearly 30 clinical readouts before end of April". BioCentury Inc.. https://www.biocentury.com/article/304658.
- ↑ COVID-19 Clinical Research Coalition (April 2020). "Global coalition to accelerate COVID-19 clinical research in resource-limited settings". Lancet 395 (10233): 1322–1325. doi:10.1016/s0140-6736(20)30798-4. PMID 32247324. PMC 7270833. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30798-4/fulltext#articleInformation.
- ↑ "A living systematic review protocol for COVID-19 clinical trial registrations". Wellcome Open Research 5: 60. 2 April 2020. doi:10.12688/wellcomeopenres.15821.1. PMID 32292826.
- ↑ "UN health chief announces global 'solidarity trial' to jumpstart search for COVID-19 treatment". 18 March 2020. https://news.un.org/en/story/2020/03/1059722.
- ↑ "Coronavirus (COVI D-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment". U.S. Food and Drug Administration (FDA) (Press release). 4 May 2020. Retrieved 8 June 2020.
- ↑ Beeching, Nicholas J.; Fletcher, Tom E.; Fowler, Robert (2020). "BMJ Best Practices: COVID-19". BMJ. https://bestpractice.bmj.com/topics/en-gb/3000168/pdf/3000168/COVID-19.pdf. Retrieved 11 March 2020.
- ↑ Seley-Radtke, Katherine (3 April 2020). "Professor of Chemistry and Biochemistry and President-Elect of the International Society for Antiviral Research, University of Maryland, Baltimore County". The Conversation. https://theconversation.com/a-small-trial-finds-that-hydroxychloroquine-is-not-effective-for-treating-coronavirus-135484.
- ↑ "No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection" (in fr). Medecine et Maladies Infectieuses 50 (4): 384. June 2020. doi:10.1016/j.medmal.2020.03.006. PMID 32240719.
- ↑ Cha, Ariana Eunjung; McGinley, Laurie. "Antimalarial drug touted by President Trump is linked to increased risk of death in coronavirus patients, study says". https://www.washingtonpost.com/health/2020/05/22/hydroxychloroquine-coronavirus-study/.
- ↑ "Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis" (in English). Lancet 0. May 2020. doi:10.1016/S0140-6736(20)31180-6. PMID 32450107. PMC 7255293. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31180-6/abstract.
- ↑ "Retraction: "Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis"". Lancet 395 (10240): 1820glish. 4 June 2020. doi:10.1016/S0140-6736(20)31324-6. PMID 32511943. PMC 7274621. https://www.thelancet.com/lancet/article/s0140673620313246.
- ↑ "Coronavirus (COVID-19) Update: FDA Warns of Newly Discovered Potential Drug Interaction That May Reduce Effectiveness of a COVID-19 Treatment Authorized for Emergency Use". U.S. Food and Drug Administration (FDA) (Press release). 15 June 2020. Retrieved 15 June 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Boseley, Sarah (16 June 202). "Recovery trial for Covid-19 treatments: what we know so far". The Guardian. https://www.theguardian.com/world/2020/jun/16/recovery-trial-for-covid-19-treatments-what-we-know-so-far. Retrieved 21 June 2020.
- ↑ "WHO welcomes preliminary results about dexamethasone use in treating critically ill COVID-19 patients". 16 June 2020. https://www.who.int/news-room/detail/16-06-2020-who-welcomes-preliminary-results-about-dexamethasone-use-in-treating-critically-ill-covid-19-patients. Retrieved 21 June 2020.
- ↑ "Q&A: Dexamethasone and COVID-19" (in en). https://www.who.int/news-room/q-a-detail/q-a-dexamethasone-and-covid-19.
- ↑ "Dexamethasone | Coronavirus Disease COVID-19" (in en). National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/dexamethasone/.
- ↑ "In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)". Clinical Infectious Diseases. March 2020. doi:10.1093/cid/ciaa237. PMID 32150618.
- ↑ Liu, Roxanne; Miller, Josh (3 March 2020). "China approves use of Roche drug in battle against coronavirus complications". Reuters. https://www.reuters.com/article/us-health-coronavirus-china-roche-hldg/china-approves-use-of-roche-arthritis-drug-for-coronavirus-patients-idUSKBN20R0LF.
- ↑ Effective Treatment of Severe COVID-19 Patients with Tocilizumab. ChinaXiv.org. 5 March 2020. doi:10.12074/202003.00026. http://chinaxiv.org/abs/202003.00026. Retrieved 14 March 2020.
- ↑ Ovadia, Daniela; Agenzia, Zoe. "COVID-19—Italy launches an independent trial on tocilizumab". Aptus Health. https://www.univadis.co.uk/viewarticle/covid-19-italy-launches-an-independent-trial-on-tocilizumab-715741.
- ↑ "Tocilizumab in COVID-19 Pneumonia (TOCIVID-19) (TOCIVID-19)". https://clinicaltrials.gov/ct2/show/NCT04317092.
- ↑ "How doctors can potentially significantly reduce the number of deaths from Covid-19". Vox. 12 March 2020. https://www.vox.com/2020/3/12/21176783/coronavirus-covid-19-deaths-china-treatment-cytokine-storm-syndrome.
- ↑ "Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China". Intensive Care Medicine 46 (5): 846–848. March 2020. doi:10.1007/s00134-020-05991-x. PMID 32125452.
- ↑ "COVID-19: consider cytokine storm syndromes and immunosuppression". Lancet 395 (10229): 1033–1034. March 2020. doi:10.1016/S0140-6736(20)30628-0. PMID 32192578.
- ↑ Slater, Hannah (26 March 2020). "FDA Approves Phase III Clinical Trial of Tocilizumab for COVID-19 Pneumonia". Cancer Network. https://www.cancernetwork.com/news/fda-approves-phase-iii-clinical-trial-tocilizumab-covid-19-pneumonia.
- ↑ "Preliminary Results of Prophylactic Tocilizumab after Axicabtageneciloleucel (axi-cel; KTE-C19) Treatment for Patients with Refractory,Aggressive Non-Hodgkin Lymphoma (NHL)". Blood 130 (Supplement 1): 1547. 2017. doi:10.1182/blood.V130.Suppl_1.1547.1547. https://ashpublications.org/blood/article/130/Supplement%201/1547/79746.
- ↑ "GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts". Blood 133 (7): 697–709. February 2019. doi:10.1182/blood-2018-10-881722. PMID 30463995.
- ↑ "Northwell Health Initiates Clinical Trials of 2 COVID-19 Drugs". 21 March 2020. https://www.longislandpress.com/2020/03/21/northwell-health-initiates-clinical-trials-of-2-covid-19-drugs/.
- ↑ 363.0 363.1 363.2 363.3 "The convalescent sera option for containing COVID-19". The Journal of Clinical Investigation 130 (4): 1545–1548. April 2020. doi:10.1172/JCI138003. PMID 32167489.
- ↑ 364.0 364.1 Piechotta, Vanessa; Chai, Khai Li; Valk, Sarah J.; Doree, Carolyn; Monsef, Ina; Wood, Erica M.; Lamikanra, Abigail; Kimber, Catherine et al. (2020-07-10). "Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review". The Cochrane Database of Systematic Reviews 7: CD013600. doi:10.1002/14651858.CD013600.pub2. ISSN 1469-493X. PMID 32648959. https://www.ncbi.nlm.nih.gov/pubmed/32648959.
- ↑ 365.0 365.1 Ho, Mitchell (2020). "Perspectives on the development of neutralizing antibodies against SARS-CoV-2". Antibody Therapeutics 3 (2): 109–114. doi:10.1093/abt/tbaa009. PMID 32566896.
- ↑ Pearce, Katie (13 March 2020). "Antibodies from COVID-19 survivors could be used to treat patients, protect those at risk: Infusions of antibody-laden blood have been used with reported success in prior outbreaks, including the SARS epidemic and the 1918 flu pandemic". The Hub at Johns Hopkins University. https://hub.jhu.edu/2020/03/13/covid-19-antibody-sera-arturo-casadevall/.
External links
Health agencies
- Coronavirus disease (COVID-19) by the World Health Organization (WHO)
- Coronavirus 2019 (COVID-19) by the US Centers for Disease Control and Prevention (CDC)
- Coronavirus (COVID-19) by the UK National Health Service (NHS)
Directories
Medical journals
- Coronavirus Disease 2019 (COVID-19) by JAMA
- Coronavirus: News and Resources by the BMJ Publishing Group
- Novel Coronavirus Information Center by Elsevier
- COVID-19 Resource Centre by The Lancet
- SARS-CoV-2 and COVID-19 by Nature
- Coronavirus (Covid-19) by The New England Journal of Medicine
- Covid-19: Novel Coronavirus by Wiley Publishing
Treatment guidelines
- "JHMI Clinical Recommendations for Available Pharmacologic Therapies for COVID-19" (PDF). https://www.hopkinsguides.com/hopkins/ub?cmd=repview&type=479-1151&name=13_538747_PDF.
- "Bouncing Back From COVID-19: Your Guide to Restoring Movement". https://www.hopkinsmedicine.org/physical_medicine_rehabilitation/coronavirus-rehabilitation/_files/impact-of-covid-patient-recovery.pdf.
- "Guidelines on the Treatment and Management of Patients with COVID-19". https://www.idsociety.org/globalassets/idsa/practice-guidelines/covid-19/treatment/idsa-covid-19-gl-tx-and-mgmt-v2.1.0.pdf.
- "Coronavirus Disease 2019 (COVID-19) Treatment Guidelines". https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf.
| Classification |
|---|