Medicine:Rh blood group system

The Rh blood group system is a human blood group system. It contains proteins on the surface of red blood cells. After the ABO blood group system, it is the most likely to be involved in transfusion reactions. The Rh blood group system consisted of 49 defined blood group antigens[1] in 2005. As of 2023, there are over 50 antigens among which the five antigens D, C, c, E, and e are the most important. There is no d antigen. Rh(D) status of an individual is normally described with a positive (+) or negative (−) suffix after the ABO type (e.g., someone who is A+ has the A antigen and Rh(D) antigen, whereas someone who is A− has the A antigen but lacks the Rh(D) antigen). The terms Rh factor, Rh positive, and Rh negative refer to the Rh(D) antigen only. Antibodies to Rh antigens can be involved in hemolytic transfusion reactions and antibodies to the Rh(D) and Rh antigens confer significant risk of hemolytic disease of the newborn.
Nomenclature
| Fisher–Race | Wiener |
|---|---|
| Dce | R0 |
| DCe | R1 |
| DcE | R2 |
| DCE | RZ |
| dce | r |
| dCe | r' |
| dcE | r″ |
| dCE | ry |
The Rh blood group system has two sets of nomenclatures: one developed by Ronald Fisher and R. R. Race, the other by Wiener. Both systems reflected alternative theories of inheritance. The Fisher–Race system, which is more commonly in use today, uses the CDE nomenclature. This system was based on the theory that a separate gene controls the product of each corresponding antigen (e.g., a "D gene" produces D antigen, and so on). However, the d gene was hypothetical, not actual.
The Wiener system used the Rh–Hr nomenclature. This system was based on the theory that there was one gene at a single locus on each of the two copies of chromosome 1, each contributing to production of multiple antigens. In this theory, a gene R1 is supposed to give rise to the "blood factors" Rh0, rh′, and rh″ (corresponding to modern nomenclature of the D, C, and E antigens) and the gene r to produce hr′ and hr″ (corresponding to modern nomenclature of the c and e antigens).[3]
Notations of the two theories are used interchangeably in blood banking (e.g., Rho(D) meaning RhD positive). Wiener's notation is more complex and cumbersome for routine use. Because it is simpler to explain, the Fisher–Race theory has become more widely used.[citation needed]
DNA testing has shown that both are partially correct: There are in fact two linked genes, the RHD gene which produces a single immune specificity (anti-D) and the RHCE gene with multiple specificities (anti-C, anti-c, anti-E, anti-e). Thus, Wiener's postulate that a gene could have multiple specificities (something many did not give credence to originally) has been proved to be correct. On the other hand, Wiener's theory that there is only one gene has proved to be incorrect, as has the Fisher–Race theory that there are three genes, rather than the two. The CDE notation used in the Fisher–Race nomenclature is sometimes rearranged to DCE to more accurately represent the co-location of the C and E encoding on the RhCE gene, and to make interpretation easier.[citation needed]
Antigens
The proteins which carry the Rh antigens are transmembrane proteins, whose structure suggests that they are ion channels.[4] The main antigens are D, C, E, c and e, which are encoded by two adjacent gene loci, the RHD gene which encodes the RhD protein with the D antigen (and variants)[5] and the RHCE gene which encodes the RhCE protein with the C, E, c and e antigens (and variants).[6] There is no d antigen. Lowercase "d" indicates the absence of the D antigen (the gene is usually deleted or otherwise nonfunctional).[citation needed]
Rh phenotypes are readily identified through the presence or absence of the Rh surface antigens. As can be seen in the table below, most of the Rh phenotypes can be produced by several different Rh genotypes. The exact genotype of any individual can only be identified by DNA analysis. Regarding patient treatment, only the phenotype is usually of any clinical significance to ensure a patient is not exposed to an antigen they are likely to develop antibodies against. A probable genotype may be speculated on, based upon the statistical distributions of genotypes in the patient's place of origin.[citation needed]
R0 (cDe or Dce) is today most common in Africa. The allele was thus often assumed in early blood group analyses to have been typical of populations on the continent; particularly in areas below the Sahara. Ottensooser et al. (1963) suggested that high R0 frequencies were likely characteristic of the ancient Judean Jews, who had emigrated from Egypt prior to their dispersal throughout the Mediterranean Basin and Europe[7] on the basis of high R0 percentages among Sephardi and Ashkenazi Jews compared to native European populations and the relative genetic isolation of Ashkenazim. However, more recent studies have found R0 frequencies as low as 24.3% among some Afroasiatic-speaking groups in the Horn of Africa,[8] as well as higher R0 frequencies among certain other Afroasiatic speakers in North Africa (37.3%)[9] and among some Palestinians in the Levant (30.4%).[10] On the contrary, at a frequency of 47.2% of the population of Basque country having the lack of the D antigen, these people display the highest frequency of the Rh negative phenotype.[11]
| Phenotype expressed on cell | Genotype expressed in DNA | Prevalence (%) | |
|---|---|---|---|
| Fisher–Race notation | Wiener notation | ||
| D+ C+ E+ c+ e+ (RhD+) | Dce/DCE | R0RZ | 0.0125 |
| Dce/dCE | R0rY | 0.0003 | |
| DCe/DcE | R1R2 | 11.8648 | |
| DCe/dcE | R1r″ | 0.9992 | |
| DcE/dCe | R2r′ | 0.2775 | |
| DCE/dce | RZr | 0.1893 | |
| D+ C+ E+ c+ e− (RhD+) | DcE/DCE | R2RZ | 0.0687 |
| DcE/dCE | R2rY | 0.0014 | |
| DCE/dcE | RZr″ | 0.0058 | |
| D+ C+ E+ c− e+ (RhD+) | DCe/dCE | R1rY | 0.0042 |
| DCE/dCe | RZr′ | 0.0048 | |
| DCe/DCE | R1RZ | 0.2048 | |
| D+ C+ E+ c− e− (RhD+) | DCE/DCE | RZRZ | 0.0006 |
| DCE/dCE | RZrY | < 0.0001 | |
| D+ C+ E− c+ e+ (RhD+) | Dce/dCe | R0r′ | 0.0505 |
| DCe/dce | R1r | 32.6808 | |
| DCe/Dce | R1R0 | 2.1586 | |
| D+ C+ E− c− e+ (RhD+) | DCe/DCe | R1R1 | 17.6803 |
| DCe/dCe | R1r′ | 0.8270 | |
| D+ C− E+ c+ e+ (RhD+) | DcE/Dce | R2R0 | 0.7243 |
| Dce/dcE | R0r″ | 0.0610 | |
| DcE/dce | R2r | 10.9657 | |
| D+ C− E+ c+ e− (RhD+) | DcE/DcE | R2R2 | 1.9906 |
| DcE/dcE | R2r″ | 0.3353 | |
| D+ C− E− c+ e+ (RhD+) | Dce/Dce | R0R0 | 0.0659 |
| Dce/dce | R0r | 1.9950 | |
| D− C+ E+ c+ e+ (RhD−) | dce/dCE | rrY | 0.0039 |
| dCe/dcE | r′r″ | 0.0234 | |
| D− C+ E+ c+ e− (RhD−) | dcE/dCE | r″rY | 0.0001 |
| D− C+ E+ c− e+ (RhD−) | dCe/dCE | r′rY | 0.0001 |
| D− C+ E+ c− e− (RhD−) | dCE/dCE | rYrY | < 0.0001 |
| D− C+ E− c+ e+ (RhD−) | dce/dCe | rr′ | 0.7644 |
| D− C+ E− c− e+ (RhD−) | dCe/dCe | r′r′ | 0.0097 |
| D− C− E+ c+ e+ (RhD−) | dce/dcE | rr″ | 0.9235 |
| D− C− E+ c+ e− (RhD−) | dcE/dcE | r″r″ | 0.0141 |
| D− C− E− c+ e+ (RhD−) | dce/dce | rr | 15.1020 |
• Figures taken from a study performed in 1948 on a sample of 2000 people in the United Kingdom.[12]
| Rh Phenotype | CDE | Patients (%) | Donors (%) |
|---|---|---|---|
| R1r | CcDe | 37.4 | 33.0 |
| R1R2 | CcDEe | 35.7 | 30.5 |
| R1R1 | CDe | 5.7 | 21.8 |
| rr | ce | 10.3 | 11.6 |
| R2r | cDEe | 6.6 | 10.4 |
| R0R0 | cDe | 2.8 | 2.7 |
| R2R2 | cDE | 2.8 | 2.4 |
| rr″ | cEe | – | 0.98 |
| RZRZ | CDE | – | 0.03 |
| rr′ | Cce | 0.8 | – |
Rh antibodies
Rh antibodies are Immunoglobulin G (IgG) antibodies which are acquired through exposure to Rh-positive blood (generally either through pregnancy or transfusion of blood products). The D antigen is the most immunogenic of all the non-ABO antigens. Approximately 80% of individuals who are D-negative and exposed to a single D-positive unit will produce an anti-D antibody. The percentage of alloimmunization is significantly reduced in patients who are actively exsanguinating.[14]
All Rh antibodies except D display dosage (antibody reacts more strongly with red cells homozygous for an antigen than cells heterozygous for the antigen (EE stronger reaction vs Ee)).
If anti-E is detected, the presence of anti-c should be strongly suspected (due to combined genetic inheritance). It is therefore common to select c-negative and E-negative blood for transfusion patients who have an anti-E and lack the c antigen (in general, a patient will not produce antibodies against their own antigens). Anti-c is a common cause of delayed hemolytic transfusion reactions.[15]
Hemolytic disease of the newborn
The hemolytic condition occurs when there is an incompatibility between the blood types of the mother and fetus. There is also potential incompatibility if the mother is Rh negative and the father is positive. When the mother conceives for the first time, with a positive child, she will become extremely sensitive. When any incompatibility is detected when she conceives the second time in less than two years then, the mother often receives an injection at 28 weeks gestation and at birth to avoid the development of antibodies towards the fetus. If not given, then the baby will be dead and must be aborted. These terms do not indicate which specific antigen-antibody incompatibility is implicated. The disorder in the fetus due to Rh D incompatibility is known as erythroblastosis fetalis.
- Hemolytic comes from two words: "hema" (blood) and "lysis" (solution) or breaking down of red blood cells
- Erythroblastosis refers to the making of immature red blood cells
- Fetalis refers to the fetus.
When the condition is caused by the Rh D antigen-antibody incompatibility, it is called Rh D Hemolytic disease of the newborn or Rh disease. Here, sensitization to Rh D antigens (usually by feto-maternal transfusion during pregnancy) may lead to the production of maternal IgG anti-D antibodies which can pass through the placenta. This is of particular importance to D negative females at or below childbearing age, because any subsequent pregnancy may be affected by the Rh D hemolytic disease of the newborn if the baby is D positive. The vast majority of Rh disease is preventable in modern antenatal care by injections of IgG anti-D antibodies (Rho(D) Immune Globulin). The incidence of Rh disease is mathematically related to the frequency of D negative individuals in a population, so Rh disease is rare in old-stock populations of Africa and the eastern half of Asia, and the Indigenous peoples of Oceania and the Americas, but more common in other genetic groups, most especially Western Europeans, but also other West Eurasians, and to a lesser degree, native Siberians, as well as those of mixed-race with a significant or dominant descent from those (e.g. the vast majority of Latin Americans and Central Asians).
- Symptoms and signs in the fetus:
- Enlarged liver, spleen, or heart and fluid buildup in the fetus' abdomen seen via ultrasound.
- Symptoms and signs in the newborn:
- Anemia that creates the newborn's pallor (pale appearance).
- Jaundice or yellow discoloration of the newborn's skin, sclera or mucous membrane. This may be evident right after birth or after 24–48 hours after birth. This is caused by bilirubin (one of the end products of red blood cell destruction).
- Enlargement of the newborn's liver and spleen.
- The newborn may have severe edema of the entire body.
- Dyspnea (difficulty breathing)
Other animals with Rh-like antigens causing hemolytic disease of the newborn
Rh disease only occurs in human fetuses however a similar disease called Neonatal isoerythrolysis (NI) can be observed in animal species of newborn horses, mules, pigs, cats, cattle, and dogs. What differs between Rh disease and NI is the pathogenesis of hemolysis between human fetuses and the animal species. With human mothers, the maternal antibodies are formed from the sensitization of foreign antigens of her unborn fetus’s red blood cells passing through the placenta causing hemolysis before birth, with other animals however, these maternal antibodies are not passed through the placenta but through colostrum. The newborn animal is without NI but soon develops hemolytic anemia after initial ingestion of its mother’s colostrum that contain antibodies that can be absorbed through the newborn’s intestines and are incompatible to its red blood cell antigen. After 48 hours of birth, the newborn may be allowed to nurse from its mother as her antibodies can no longer be absorbed through the neonate’s intestines. Because the most active newborn animals consume the most colostrum, they may be the ones who are most affected by the blood incompatibility of antigen and antibody.[16]
Rh proteins outside of human species
Rh molecules can be found in many different living organisms from worms, bacteria, algae, and other vertebrates. These Rh molecules from different animals have the same biochemical function-differing slightly in their amino acid sequences.[17] The Rh proteins in other species, however, do not correspond with the Rh blood group or antigens found on human red blood cells. One such example would be the nematode Caenorhabditis elegans. Because this worm does not have red blood cells, it cannot have Rh antigens, excluding it from having a Rh blood type. These Rh proteins therefore do not bind to red blood cells; they operate independently. Instead of transporting CO
2 from the proteins of human red blood cells, C. elegan’s Rh proteins transport NH3 out of its body.[18]
Population data
According to a comprehensive study, the worldwide frequency of Rh-positive and Rh-negative blood types is approximately 00% and 00%, respectively. The same study concluded that the share of the population with Rh-negative blood type is set to fall further in the future primarily due to low population growth in Europe.[19] The frequency of Rh factor blood types and the RhD neg allele gene differs in various populations.[citation needed]
| Population | Rh(D) Neg | Rh(D) Pos | Rh(D) Neg alleles |
|---|---|---|---|
| African Americans | ~ 7% | 93% | ~ 26% |
| Albania[21] | 10.86% | 89% | weak D 1.4% |
| Basques[22] | 21%–36% | 65% | ~ 60% |
| United Kingdom [23] | 17% | 83% | |
| China [23] | < 1% | > 99% | |
| Ethiopians[23] | 1%–21% | 99%–79% | |
| Europeans (others) | 16% | 84% | 40% |
| India [23] | 0.6%–8.4% | 99.4%–91.6% | |
| Indonesia[23] | < 1% | > 99% | |
| Japan [23] | < 1% | > 99% | |
| Koreans[24] | < 1% | > 99% | |
| Madagascar [23] | 1% | 99% | |
| Moroccans[25] | 9.5% | 90.5% | |
| Moroccans (High Atlas)[26] | ~ 29% | 71% | |
| Native Americans | ~ 1% | 99% | ~ 10% |
| Nigeria[27] | 6% | 94% | |
| Saudi Arabia[28] | 8.8% | 91.2% | 29.5% |
| Subequatorial Africa[23] | 1%–3% | 99%–97% | |
| United States [23] | 15% | 85% |
Genetics
The D antigen is inherited as one gene (RHD) (on the short arm of the first chromosome, p36.13–p34.3) with various alleles. Typically, Rhesus positive people have an intact RHD gene while negative people lack the gene (or have mutations in it). However, there are exceptions: for instance, Japanese and black Africans may have an intact gene that is not expressed or only at very low levels.[29] The gene codes for the RhD protein on the red blood cell membrane. D− individuals who lack a functional RHD gene do not produce the D antigen, and may be immunized by D+ blood.[citation needed]
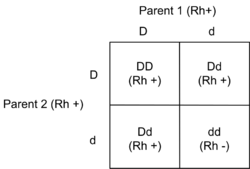
The D antigen is a dominant trait. If both of a child's parents are Rh negative, the child will definitely be Rh negative. Otherwise the child may be Rh positive or Rh negative, depending on the parents' specific genotypes.[30]
The epitopes for the next 4 most common Rh antigens, C, c, E and e are expressed on the highly similar RhCE protein that is genetically encoded in the RHCE gene, also found on chromosome 1. It has been shown that the RHD gene arose by duplication of the RHCE gene during primate evolution. Mice have just one RH gene.[31]
The RHAG gene, which is responsible for encoding Rh-associated glycoprotein (RhAG), is found on chromosome 6a.
The polypeptides produced from the RHD and RHCE genes form a complex on the red blood cell membrane with the Rh-associated glycoprotein.[15]
Function
| Blood group Rh C/E/D polypeptide | |
|---|---|
| Identifiers | |
| Symbol | ? |
| InterPro | IPR002229 |
On the basis of structural homology it has been proposed that the product of RHD gene, the RhD protein, is a membrane transport protein of uncertain specificity (CO2 or NH3) and unknown physiological role.[32][33] The three-dimensional structure of the related RHCG protein and biochemical analysis of the RhD protein complex indicates that the RhD protein is one of three subunits of an ammonia transporter.[34][35] Three recent studies[36][37][38] have reported a protective effect of the RhD-positive phenotype, especially RhD heterozygosity, against the negative effect of latent toxoplasmosis on psychomotor performance in infected subjects. RhD-negative compared to RhD-positive subjects without anamnestic titres of anti-Toxoplasma antibodies have shorter reaction times in tests of simple reaction times. And conversely, RhD-negative subjects with anamnestic titres (i.e. with latent toxoplasmosis) exhibited much longer reaction times than their RhD-positive counterparts. The published data suggested that only the protection of RhD-positive heterozygotes was long term in nature; the protection of RhD-positive homozygotes decreased with duration of the infection while the performance of RhD-negative homozygotes decreased immediately after the infection. The overall change in reaction times was always larger in the RhD-negative group than in the RhD-positive.[citation needed]
RHD polymorphism
Origin of RHD polymorphism
For a long time, the origin of RHD polymorphism was an evolutionary enigma.[39][40][41] Before the advent of modern medicine, the carriers of the rarer allele (e.g. RhD-negative women in a population of RhD positives or RhD-positive men in a population of RhD negatives) were at a disadvantage as some of their children (RhD-positive children born to preimmunised RhD-negative mothers) were at a higher risk of fetal or newborn death or health impairment from hemolytic disease.[citation needed]
Natural selection aside, the RHD-RHCE region is structurally predisposed to many mutations seen in humans, since the pair arose by gene duplication and remain similar enough for unequal crossing over to occur.[31] In addition to the case where D is deleted, crossover can also produce a single gene mixing exons from both RHD and RHCE, forming the majority of partial D types.[42]:323
Weak D
| Weak D | Partial D | |
|---|---|---|
| Change in D | Decreased amount | Structural alternation |
| Can donate as if being: |
D positive | D positive |
| Can receive blood as if being: |
D positive (usually)[42] | D negative[15] |
In serologic testing, D positive blood is easily identified. Units that are D negative are often retested to rule out a weaker reaction. This was previously referred to as Du, which has been replaced.[42]:322 By definition, weak D phenotype is characterized by negative reaction with anti-D reagent at immediate spin (IS), negative reaction after 37 °C incubation, and positive reaction at anti-human globulin (AHG) phase. Weak D phenotype can occur in several ways. In some cases, this phenotype occurs because of an altered surface protein that is more common in people of European descent. An inheritable form also occurs, as a result of a weakened form of the R0 gene. Weak D may also occur as "C in trans", whereby a C gene is present on the opposite chromosome to a D gene (as in the combination R0r', or "Dce/dCe"). The testing is difficult, since using different anti-D reagents, especially the older polyclonal reagents, may give different results.
The practical implication of this is that people with this sub-phenotype will have a product labeled as "D positive" when donating blood. When receiving blood, they are sometimes typed as a "D negative", though this is the subject of some debate. Most "Weak D" patients can receive "D positive" blood without complications.[42]:323 However, it is important to correctly identify the ones that have to be considered D+ or D−. This is important, since most blood banks have a limited supply of "D negative" blood and the correct transfusion is clinically relevant. In this respect, genotyping of blood groups has much simplified this detection of the various variants in the Rh blood group system.
Partial D
It is important to differentiate weak D (due to a quantitative difference in the D antigen) from partial D (due to a qualitative difference in the D antigen). Simply put, the weak D phenotype is due to a reduced number of D antigens on a red blood cell. In contrast, the partial D phenotype is due to an alteration in D-epitopes. Thus, in partial D, the number of D antigens is not reduced but the protein structure is altered. These individuals, if alloimmunized to D, can produce an anti-D antibody. Therefore, partial D patients who are donating blood should be labeled as D-positive but, if receiving blood, they should be labeled as D-negative and receive D-negative units.[15]
In the past, partial D was called 'D mosaic' or 'D variant.' Different partial D phenotypes are defined by different D epitopes on the outer surface of the red blood cell membrane. More than 30 different partial D phenotypes have been described.[15]
Rhnull phenotype
Rhnull individuals have no Rh antigens (no Rh or RhAG) on their red blood cells.[43] This rare condition[43] has been called "Golden Blood".[44] As a consequence of Rh antigen absence, Rhnull red blood cells also lack LW and Fy5 and show weak expression of S, s, and U antigens.
Red blood cells lacking Rh/RhAG proteins have structural abnormalities (such as stomatocytosis) and cell membrane defects that can result in hemolytic anemia.[15][43]
The first Rhnull blood was discovered in an Aboriginal Australian woman, in 1961.[45] Only 43 individuals have been reported to have it worldwide. Only nine active donors have been reported.[44] Its properties make it attractive in numerous medical applications, but scarcity makes it expensive to transport and acquire.[46]
Other Rh group antigens
As of 2023, over 50 antigens have been described in the Rh group system; among those described here, the D, C, c, E and e antigens are the most important. The others are much less frequently encountered or are rarely clinically significant. Each is given a number, though the highest assigned number (CEVF or RH61 according to the ISBT terminology) is not an accurate reflection of the antigens encountered since many (e.g. Rh38) have been combined, reassigned to other groups, or otherwise removed.[42]:324
Some of the other Rh "antigens" are f ("ce", RH6), Ce (RH7), Cw (RH8), Cx (RH9), V (RH10), Ew (RH11), G (RH12), Tar (RH40), VS (RH20), Dw (RH23), and CE (RH22). Some of these groups, including f, Ce and CE, describe grouping of some existing groups. Others, like V, describe an epitope created by some other mutation on the RHD and RHCE genes. V in particular is caused by a mutation on RHCE.[47]
History
The term "Rh" was originally an abbreviation of "Rhesus factor". It was discovered in 1939 by Karl Landsteiner and Alexander S. Wiener, who, at the time, believed it to be a similar antigen found in rhesus macaque red blood cells. It was subsequently discovered that the human factor is not identical to the rhesus monkey factor, but by then, "Rhesus Group" and like terms were already in widespread, worldwide use. Thus, notwithstanding it is a misnomer, the term survives (e.g., rhesus blood group system and the obsolete terms rhesus factor, rhesus positive, and rhesus negative – all three of which actually refer specifically and only to the Rh D factor and are thus misleading when unmodified). Contemporary practice is to use "Rh" as a term of art instead of "Rhesus" (e.g., "Rh Group", "Rh factors", "Rh D", etc.).
The significance of their discovery was not immediately apparent and was only realized in 1940, after subsequent findings by Philip Levine and Rufus Stetson.[48] The serum that led to the discovery was produced by immunizing rabbits with red blood cells from a rhesus macaque. The antigen that induced this immunization was designated by them as Rh factor to indicate that rhesus blood had been used for the production of the serum.[49]
In 1939, Phillip Levine and Rufus Stetson published in a first case report the clinical consequences of non-recognized Rh factor, hemolytic transfusion reaction, and hemolytic disease of the newborn in its most severe form.[50] It was recognized that the serum of the reported woman agglutinated with red blood cells of about 80% of the people although the then known blood groups, in particular ABO were matched. No name was given to this agglutinin when described. In 1940, Landsteiner and Wiener made the connection to their earlier discovery, reporting a serum that also reacted with about 85% of different human red blood cells.[51]
In 1941, Group O: a patient in Irvington, New Jersey, US, delivered a normal[clarification needed] infant in 1931; this pregnancy was followed by a long period of sterility. The second pregnancy (April, 1941) resulted in an infant with icterus gravis.[52] In May 1941, the third anti-Rh serum (M.S.) of Group O became available.[52]
Based on the serologic similarities, 'Rh factor' was later also used for antigens, and anti-Rh for antibodies, found in humans such as those previously described by Levine and Stetson. Although differences between these two sera were shown already in 1942 and clearly demonstrated in 1963, the already widely used term "Rh" was kept for the clinically described human antibodies which are different from the ones related to the rhesus monkey. This real factor found in rhesus macaque was classified in the Landsteiner-Weiner antigen system (antigen LW, antibody anti-LW) in honor of the discoverers.[53][54]
It was recognized that the Rh factor was just one in a system of various antigens. Based on different models of genetic inheritance, two different terminologies were developed; both of them are still in use.
The clinical significance of this highly immunizing D antigen (i.e., Rh factor) was soon realized. Some keystones were to recognize its importance for blood transfusion (including reliable diagnostic tests), hemolytic disease of the newborn (including exchange transfusion), and very importantly the prevention of it by screening and prophylaxis.
The discovery of cell-free fetal DNA in maternal circulation by Holzgrieve et al. led to the noninvasive genotyping of fetal Rh genes in many countries.
References
- ↑ Dean, Laura. Blood Groups and Red Cell Antigens [Internet].. Bethesda (MD): National Center for Biotechnology Information (US); 2005, Chapter. 7.
- ↑ "Rh System". https://learnserology.ca/LearnSerology_content/LS3_Rh_System.html.
- ↑ "Genetics and Nomenclature of the Rh–Hr Blood Types". Antonie van Leeuwenhoek 15 (1): 17–28. 1 February 1949. doi:10.1007/BF02062626. ISSN 0003-6072.
- ↑ "dbRBC – Blood Group Antigen Gene Mutation Database". www.ncbi.nlm.nih.gov. https://www.ncbi.nlm.nih.gov/gv/mhc/xslcgi.cgi?cmd=bgmut/systems_info&system=rh.
- ↑ "RHD Rh blood group, D antigen [Homo sapiens – Gene Result"]. nlm.nih.gov. https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=6007.
- ↑ "RHCE Rh blood group, CcEe antigens [Homo sapiens – Gene Result"]. nlm.nih.gov. https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=6006&ordinalpos=1&itool=EntrezSystem2.PEntrez.Gene.Gene_ResultsPanel.Gene_RVDocSum.
- ↑ "Blood groups of a population of Ashkenazi Jews in Brazil". American Journal of Physical Anthropology 21 (1): 41–8. March 1963. doi:10.1002/ajpa.1330210106. PMID 13940710.
- ↑ "The effects of altitudinal variation in Ethiopian populations". Philosophical Transactions of the Royal Society of London B: Biological Sciences 256 (805): 147–182. 1969. doi:10.1098/rstb.1969.0040. Bibcode: 1969RSPTB.256..147H.
- ↑ "A genetic study of nine populations from the region of Tlemcen in Western Algeria: a comparative analysis on the Mediterranean scale". Anthropological Science 120 (3): 209–216. 2012. doi:10.1537/ase.120618. https://www.jstage.jst.go.jp/article/ase/120/3/120_120618/_html. Retrieved 28 August 2017.
- ↑ "The Rh allele frequencies in Gaza city in Palestine". Asian Journal of Transfusion Science 5 (2): 150–2. July 2011. doi:10.4103/0973-6247.83241. PMID 21897594.
- ↑ "Sequence diversity of the Rh blood group system in Basques". European Journal of Human Genetics 26 (12): 1859–1866. December 2018. doi:10.1038/s41431-018-0232-1. PMID 30089826.
- ↑ "The Rh chromosome frequencies in England". Blood 3 (6): 689–95. June 1948. doi:10.1182/blood.V3.6.689.689. PMID 18860341.
- ↑ "Rh Subgroups and Kell Antigens in Patients With Thalassemia and in Donors in Turkey". Turkish Journal of Medical Sciences 29: 155–7. 1999. https://journals.tubitak.gov.tr/medical/issues/sag-99-29-2/sag-29-2-15-98073.pdf. Retrieved 2008-10-17.
- ↑ Roback et al. AABB Technical Manual, 16th Ed. Bethesda, AABB Press, 2008.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 Mais, DD. ASCP Quick Compendium of Clinical Pathology, 2nd Ed. Chicago, ASCP Press, 2009.
- ↑ Hematology. Quick Look Series (1st ed.). Teton NewMedia. June 1, 2001. pp. 32–33. ISBN 978-1893441361.
- ↑ "CeRh1 (rhr-1) is a dominant Rhesus gene essential for embryonic development and hypodermal function in Caenorhabditis elegans". Proceedings of the National Academy of Sciences of the United States of America 103 (15): 5881–5886. April 2006. doi:10.1073/pnas.0600901103. PMID 16595629. Bibcode: 2006PNAS..103.5881J.
- ↑ "What is Rhesus factor? Did we get it from Rhesus monkeys? Can Rh factor be found in other animals?" (in en). 2018-11-20. https://www.thetech.org/ask-a-geneticist/rhesus-factor-evolution.
- ↑ "Blood Type Frequencies by Country including the Rh Factor – Rhesus Negative". https://www.rhesusnegative.net/themission/bloodtypefrequencies/.
- ↑ "Re: Is the RH negative blood type more prevalent in certain ethnic groups?". MadSci Network. 2001-03-21. https://www.madsci.org/posts/archives/mar2001/985200157.Ge.r.html.
- ↑ "Distribution of Rhesus blood group antigens and weak D alleles in the population of Albania". Blood Transfusion = Trasfusione del Sangue 12 (4): 565–9. October 2014. doi:10.2450/2014.0240-13. PMID 24960662.
- ↑ "Distribution of rhesus blood group system in the French basques: a reappraisal using the allele-specific primers PCR method". Human Heredity 58 (2): 69–72. 2004. doi:10.1159/000083027. PMID 15711086.
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 23.6 23.7 23.8 "High rhesus (Rh(D)) negative frequency and ethnic-group based ABO blood group distribution in Ethiopia". BMC Research Notes 10 (1): 330. July 2017. doi:10.1186/s13104-017-2644-3. PMID 28747227.
- ↑ "Molecular characterization of D- Korean persons: development of a diagnostic strategy". Transfusion 45 (3): 345–52. March 2005. doi:10.1111/j.1537-2995.2005.04311.x. PMID 15752151.
- ↑ "Prevalence of weak D phenotype among D negative C/E+ blood donors in Morocco". International Journal of Blood Transfusion and Immunohematology 6 (1): 3–7. 2016. doi:10.5348/ijbti-2016-22-OA-2. https://www.ijbti.com/archive/2016-archive/100022IJBTITO2016-wafi/100022IJBTITO2016-wafi.pdf. Retrieved February 3, 2018.
- ↑ "It is worthwhile filling in the remaining blank spots for blood group antigen frequencies". Blood Transfusion = Trasfusione del Sangue 12 (1): 3–6. January 2014. doi:10.2450/2013.0192-13. PMID 24120599.
- ↑ "Distribution of ABO and Rh-D blood groups in the Benin area of Niger-Delta: Implication for regional blood transfusion". Asian Journal of Transfusion Science 2 (1): 3–5. January 2008. doi:10.4103/0973-6247.39502. PMID 20041069.
- ↑ "Distribution of ABO and Rhesus (RHD) Blood Groups in Al-Jouf Province of the Saudi Arabia". The Anthropologist 13 (2): 99–102. April 2011. doi:10.1080/09720073.2011.11891182. https://www.krepublishers.com/02-Journals/T-Anth/Anth-13-0-000-11-Web/Anth-13-2-000-11-Abst-Pdf/Anth-13-2-099-11-688-Eweidah-M-H/Anth-13-2-099-11-688-Eweidah-M-H-Tt.pdf. Retrieved February 3, 2018.
- ↑ "The Rh blood group system: a review". Blood 95 (2): 375–387. January 2000. doi:10.1182/blood.V95.2.375. PMID 10627438.
- ↑ "ABO inheritance patterns". Inheritance patterns of blood groups. Australian Red Cross Blood Service. https://www.transfusion.com.au/?q=node/77.
- ↑ 31.0 31.1 "RHCE represents the ancestral RH position, while RHD is the duplicated gene". Blood 99 (6): 2272–3. March 2002. doi:10.1182/blood-2001-12-0153. PMID 11902138.
- ↑ "Biological gas channels for NH3 and CO
2: evidence that Rh (Rhesus) proteins are CO
2 channels". Transfusion Clinique et Biologique 13 (1–2): 103–10. 2006. doi:10.1016/j.tracli.2006.03.001. PMID 16563833. - ↑ "Physiological role of the putative ammonium transporter RhCG in the mouse". Transfusion Clinique et Biologique 13 (1–2): 167–8. 2006. doi:10.1016/j.tracli.2006.03.003. PMID 16564721.
- ↑ "Function of human Rh based on structure of RhCG at 2.1 A". Proceedings of the National Academy of Sciences of the United States of America 107 (21): 9638–43. May 2010. doi:10.1073/pnas.1003587107. PMID 20457942. Bibcode: 2010PNAS..107.9638G.
- ↑ "The structure and function of the Rh antigen complex". Seminars in Hematology 44 (1): 42–50. January 2007. doi:10.1053/j.seminhematol.2006.09.010. PMID 17198846.
- ↑ "Toxoplasma and reaction time: role of toxoplasmosis in the origin, preservation and geographical distribution of Rh blood group polymorphism". Parasitology 135 (11): 1253–61. September 2008. doi:10.1017/S003118200800485X. PMID 18752708.
- ↑ "Neurophysiological effect of the Rh factor. Protective role of the RhD molecule against Toxoplasma-induced impairment of reaction times in women". Neuro Endocrinology Letters 29 (4): 475–81. August 2008. PMID 18766148. https://natur.cuni.cz/flegr/pdf/rh2.pdf.
- ↑ "Increased incidence of traffic accidents in Toxoplasma-infected military drivers and protective effect RhD molecule revealed by a large-scale prospective cohort study". BMC Infectious Diseases 9: 72. May 2009. doi:10.1186/1471-2334-9-72. PMID 19470165.
- ↑ "Selection against heterozygosis in Man.". Annals of Eugenics 11: 333–340. 1942. doi:10.1111/j.1469-1809.1941.tb02297.x.
- ↑ "Mutation and the Rhesus reaction". Nature 153 (3873): 106. 1944. doi:10.1038/153106b0. Bibcode: 1944Natur.153..106F.
- ↑ "Is the Rh facing a crossroad? A critique of the compensation effect.". Am Nat 87 (835): 257–261. 1953. doi:10.1086/281782.
- ↑ 42.0 42.1 42.2 42.3 42.4 Technical Manual (15th ed.). Bethesda MD: American Association of Blood Banks. 2005. ISBN 978-1-56395-196-1.
- ↑ 43.0 43.1 43.2 "RH blood group system and molecular basis of Rh-deficiency". Baillière's Best Practice & Research. Clinical Haematology 12 (4): 655–89. December 1999. doi:10.1053/beha.1999.0047. PMID 10895258.
- ↑ 44.0 44.1 "Rhnull, the Rarest Blood Type on Earth, Has Been Called the "Golden Blood"". https://curiosity.com/topics/rhnull-the-rarest-blood-type-on-earth-has-been-called-the-golden-blood-curiosity/.
- ↑ "J-STAGE". https://www.jstage.jst.go.jp/article/pjab1945/46/7/46_7_733/_pdf.
- ↑ "The man with the golden blood". Mosaic Science. https://mosaicscience.com/story/man-golden-blood/.
- ↑ "Rh System: Anti-V" (in en). 2 October 2019. https://professionaleducation.blood.ca/en/transfusion/bonnes-pratiques/pratiques-suivre-en-matiere-de-serologie/rh-system-anti-v.
- ↑ "An Agglutinable Factor in Human Blood Recognized by Immune Sera for Rhesus Blood". Exp Biol Med (Maywood) 43 (1): 223. 1940. doi:10.3181/00379727-43-11151.
- ↑ "Studies on an agglutinogen (Rh) in human blood reacting with anti-rhesus sera and with human isoantibodies". The Journal of Experimental Medicine 74 (4): 309–20. September 1941. doi:10.1084/jem.74.4.309. PMID 19871137.
- ↑ "An unusual case of intragroup agglutination". JAMA 113 (2): 126–7. 1939. doi:10.1001/jama.1939.72800270002007a.
- ↑ "An agglutinable factor in human blood recognized by immune sera for rhesus blood". Proc Soc Exp Biol Med 43: 223–4. 1940. doi:10.3181/00379727-43-11151.
- ↑ 52.0 52.1 "The role of iso-immunization in the pathogenesis of erythroblastosis fetalis" (in en). American Journal of Obstetrics and Gynecology 42 (6): 925–937. December 1941. doi:10.1016/S0002-9378(41)90260-0. ISSN 0002-9378.
- ↑ "The Rh blood group system: a review". Blood 95 (2): 375–87. January 2000. doi:10.1182/blood.V95.2.375. PMID 10627438.
- ↑ "The complexities of the Rh system". Vox Sanguinis 87 (Suppl. 1): 58–62. July 2004. doi:10.1111/j.1741-6892.2004.00431.x. PMID 15200606.
External links
 |