Physics:Isotopes of neon
From HandWiki
Short description: Nuclides with atomic number of 10 but with different mass numbers
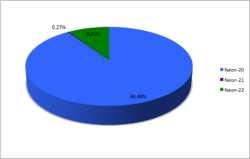
Neon (10Ne) possesses three stable isotopes: 20Ne, 21Ne, and 22Ne. In addition, 17 radioactive isotopes have been discovered, ranging from 15Ne to 34Ne, all short-lived. The longest-lived is 24Ne with a half-life of 3.38(2) min. All others are under a minute, most under a second. The least stable is 15Ne with a half-life of 770(300) ys (7.7(3.0)×10−22 s). See isotopes of carbon for notes about the measurement. Light radioactive neon isotopes usually decay to fluorine or oxygen, while heavier ones decay to sodium.
List of isotopes
| Nuclide [n 1] |
Z | N | Isotopic mass (u) [n 2][n 3] |
Half-life [resonance width] |
Decay mode [n 4] |
Daughter isotope [n 5] |
Spin and parity [n 6] |
Physics:Natural abundance (mole fraction) | |
|---|---|---|---|---|---|---|---|---|---|
| Excitation energy | Normal proportion | Range of variation | |||||||
| 15Ne[1] | 10 | 5 | 15.043170(70) | 770(300) ys [590(230) keV] |
2p | 13O | (3/2−) | ||
| 16Ne | 10 | 6 | 16.025751(22) | > 5.7 zs [< 80 keV] |
2p | 14O | 0+ | ||
| 17Ne[n 7] | 10 | 7 | 17.0177140(4) | 109.2(6) ms | β+p (94.4(2.9)%) | 16O | 1/2− | ||
| β+α (3.51(1)%) | 13N | ||||||||
| β+ (2.1(2.9)%) | 17F | ||||||||
| β+pα (0.014(4)%) | 12C | ||||||||
| 18Ne | 10 | 8 | 18.0057087(4) | 1664.20(47) ms | β+ | 18F | 0+ | ||
| 19Ne | 10 | 9 | 19.00188091(17) | 17.2569(19) s | β+ | 19F | 1/2+ | ||
| 20Ne | 10 | 10 | 19.9924401753(16) | Stable | 0+ | 0.9048(3) | [0.8847, 0.9051][2] | ||
| 21Ne | 10 | 11 | 20.99384669(4) | Stable | 3/2+ | 0.0027(1) | [0.0027, 0.0171][2] | ||
| 22Ne | 10 | 12 | 21.991385114(19) | Stable | 0+ | 0.0925(3) | [0.0920, 0.0996][2] | ||
| 23Ne | 10 | 13 | 22.99446691(11) | 37.15(3) s | β− | 23Na | 5/2+ | ||
| 24Ne | 10 | 14 | 23.9936106(6) | 3.38(2) min | β− | 24mNa | 0+ | ||
| 25Ne | 10 | 15 | 24.997810(30) | 602(8) ms | β− | 25Na | 1/2+ | ||
| 26Ne | 10 | 16 | 26.000516(20) | 197(2) ms | β− (99.87(3)%) | 26Na | 0+ | ||
| β−n (0.13(3)%) | 25Na | ||||||||
| 27Ne | 10 | 17 | 27.007570(100) | 30.9(1.1) ms | β− (98.0(5)%) | 27Na | (3/2+) | ||
| β−n (2.0(5)%) | 26Na | ||||||||
| β−2n ?[n 8] | 25Na ? | ||||||||
| 28Ne | 10 | 18 | 28.012130(140) | 18.8(2) ms | β− (84.3(1.1)%) | 28Na | 0+ | ||
| β−n (12(1)%) | 27Na | ||||||||
| β−2n (3.7(5)%) | 26Na | ||||||||
| 29Ne | 10 | 19 | 29.019750(160) | 14.7(4) ms | β− (68.0(5.1)%) | 29Na | (3/2−) | ||
| β−n (28(5)%) | 28Na | ||||||||
| β−2n (4(1)%) | 27Na | ||||||||
| 30Ne | 10 | 20 | 30.024990(270) | 7.22(18) ms | β− (78.1(4.6)%) | 30Na | 0+ | ||
| β−n (13(4)%) | 29Na | ||||||||
| β−2n (8.9(2.3)%) | 28Na | ||||||||
| 31Ne | 10 | 21 | 31.033470(290) | 3.4(8) ms | β− | 31Na | (3/2−) | ||
| β−n ?[n 8] | 30Na ? | ||||||||
| β−2n ?[n 8] | 29Na ? | ||||||||
| 32Ne | 10 | 22 | 32.039720(540)# | 3.5(9) ms | β− | 32Na | 0+ | ||
| β−n ?[n 8] | 31Na ? | ||||||||
| β−2n ?[n 8] | 30Na ? | ||||||||
| 33Ne?[n 9] | 10 | 23 | 33.049520(640)# | < 260 ns | n ?[n 8] | 32Ne | 7/2−# | ||
| 34Ne | 10 | 24 | 34.056730(550)# | 2 ms# [> 1.5 μs] | β− ?[n 8] | 34Na | 0+ | ||
| β−2n ?[n 8] | 32Ne ? | ||||||||
| β−n ?[n 8] | 33Ne ? | ||||||||
- ↑ mNe – Excited nuclear isomer.
- ↑ ( ) – Uncertainty (1σ) is given in concise form in parentheses after the corresponding last digits.
- ↑ # – Atomic mass marked #: value and uncertainty derived not from purely experimental data, but at least partly from trends from the Mass Surface (TMS).
- ↑
Modes of decay:
n: Neutron emission p: Proton emission - ↑ Bold symbol as daughter – Daughter product is stable.
- ↑ ( ) spin value – Indicates spin with weak assignment arguments.
- ↑ Has 2 halo protons.
- ↑ Jump up to: 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 8.8 Decay mode shown is energetically allowed, but has not been experimentally observed to occur in this nuclide.
- ↑ This isotope has not yet been observed; given data is inferred or estimated from periodic trends.
- The isotopic composition refers to that in air.
References
- ↑ Wamers, F.; Marganiec, J.; Aksouh, F.; Aksyutina, Yu.; Álvarez-Pol, H.; Aumann, T.; Beceiro-Novo, S.; Boretzky, K. et al. (4 April 2014). "First Observation of the Unbound Nucleus 15Ne". Physical Review Letters 112 (13): 132502. doi:10.1103/PhysRevLett.112.132502. PMID 24745409. http://publications.lib.chalmers.se/records/fulltext/198534/local_198534.pdf.
- ↑ Jump up to: 2.0 2.1 2.2 Meija, Juris; Coplen, Tyler B.; Berglund, Michael; Brand, Willi A.; Bièvre, Paul De; Gröning, Manfred; Holden, Norman E.; Irrgeher, Johanna et al. (2016-03-01). "Isotopic compositions of the elements 2013 (IUPAC Technical Report)" (in en). Pure and Applied Chemistry 88 (3): 293–306. doi:10.1515/pac-2015-0503. ISSN 1365-3075.
 |