Chemistry:2-Phenylhexane
From HandWiki
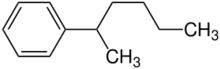
| |
| Names | |
|---|---|
| Preferred IUPAC name
(Hexan-2-yl)benzene | |
| Other names
2-Phenylhexane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H18 | |
| Molar mass | 162.276 g·mol−1 |
| Density | 0.858 g/ml |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-Phenylhexane is an aromatic hydrocarbon. It can be produced by a Friedel-Crafts alkylation between 1-chlorohexane and benzene.,[1] or by the reaction of benzene and 1-hexene with various acid catalysts such as antimony pentafluoride,[2] scandium(III) triflate,[3] and phosphoric acid.[4]
References
- ↑ Organic Chemistry Marye Anne Fox, James K. Whitesell (Google books)
- ↑ Zhurnal Organicheskoi Khimii, 17(7), 1505-11; 1981
- ↑ Choong Eui Song; Woo Ho Shimb; Eun Joo Roha; Jung Hoon Choi (2000). "Scandium(III) triflate immobilised in ionic liquids: a novel and recyclable catalytic system for Friedel-Crafts alkylation of aromatic compounds with alkenes". Chem. Commun. 2000 (17): 1695–1696. doi:10.1039/b005335j.
- ↑ Industrial & Engineering Chemistry Research, 46(9), 2902-2906; 2007
 |

