Medicine:Bronchiolitis obliterans
| Bronchiolitis obliterans[1] | |
|---|---|
| Other names | Constrictive bronchiolitis,[2] Obliterative bronchiolitis, Popcorn lung |
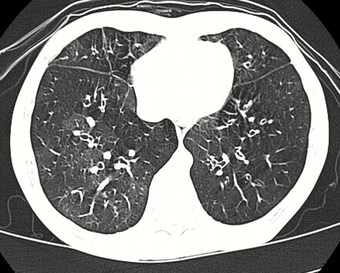 | |
| High resolution CT scan showing bronchiolitis obliterans with mosaic attenuation, bronchiectasis, air trapping and bronchial thickening[3] | |
| Symptoms | Dry cough, shortness of breath, wheezing, feeling tired[1] |
| Usual onset | Worsens over weeks to months in rare cases.[4] |
| Causes | Toxic fumes, respiratory infections, connective tissue disorder, following a bone marrow or heart-lung transplant[1] |
| Diagnostic method | CT scan, pulmonary function tests, lung biopsy[1] |
| Differential diagnosis | Asthma[5] |
| Treatment | Corticosteroids, immunosuppressive medication, lung transplant[1][4] |
| Prognosis | Often poor[4] |
| Frequency | Rare[4] |
Bronchiolitis obliterans (BO), also known as obliterative bronchiolitis, constrictive bronchiolitis and popcorn lung, is a disease that results in obstruction of the smallest airways of the lungs (bronchioles) due to inflammation.[1][6] Symptoms include a dry cough, shortness of breath, wheezing and feeling tired.[1] These symptoms generally get worse over weeks to months.[4] It is not related to cryptogenic organizing pneumonia, previously known as bronchiolitis obliterans organizing pneumonia.[4]
Causes include breathing in toxic fumes, respiratory infections, connective tissue disorder or complications following a bone marrow or heart-lung transplant.[1] Symptoms may not occur until two to eight weeks following toxic exposure or infection.[1] The underlying mechanism involves inflammation that results in scar tissue formation.[1] Diagnosis is by CT scan, pulmonary function tests or lung biopsy.[1] A chest X-ray is often normal.[4]
While the disease is not reversible, treatments can slow further worsening.[1] This may include the use of corticosteroids or immunosuppressive medication.[1] A lung transplant may be offered.[4] Outcomes are often poor, with most people dying in months to years.[4]
Bronchiolitis obliterans is rare in the general population.[4] It, however, affects about 75% of people by ten years following a lung transplant and up to 10% of people who have received a bone marrow transplant from someone else.[4] The condition was first clearly described in 1981.[4] Prior descriptions occurred as early as 1956, with the term "bronchiolitis obliterans" used first by Reynaud in 1835.[7][8]
Signs and symptoms
Bronchiolitis obliterans results in worsening shortness of breath, wheezing, and a dry cough. The symptoms can start gradually, or severe symptoms can occur suddenly.[9][10] These symptoms represent an obstructive pattern that is non-reversible with bronchodilator therapy, and need to be related to various lung insults.[11] These insults include inhalation damage, post transplant auto-immune injury, post-infectious disease, drug reactions, and several auto-immune diseases.[6]
Cause
Bronchiolitis obliterans has many possible causes, including collagen vascular disease, transplant rejection in organ transplant patients, viral infection (adenovirus, respiratory syncytial virus, influenza, HIV, cytomegalovirus), Stevens–Johnson syndrome, Pneumocystis pneumonia, drug reaction, aspiration and complications of prematurity (bronchopulmonary dysplasia), and exposure to toxic fumes. Toxins implicated in the condition include diacetyl, sulfur dioxide, nitrogen dioxide, ammonia, chlorine, thionyl chloride, methyl isocyanate, hydrogen fluoride, hydrogen bromide, hydrogen chloride, hydrogen sulfide, phosgene, polyamide-amine dyes, mustard gas and ozone.[4][6][12] It can also be present in patients with IBD, systemic lupus erythematosus, juvenile idiopathic arthritis, rheumatoid arthritis, GERD, IgA nephropathy, and ataxia telangiectasia.[13][14][6] Activated charcoal has been known to cause it when aspirated.[15] The ingestion of large doses of papaverine in the vegetable Sauropus androgynus has caused it.[16] Additionally, the disorder may be idiopathic (without known cause).[17][18][19]
Lung transplant
Bronchiolitis obliterans is a common complication in lung transplants because transplanted lungs are at greater risk of alloimmunization as compared to healthy lungs. The disease is often termed bronchiolitis obliterans syndrome (BOS) in the setting of post lung transplantation and hematopoietic stem cell transplant (HSCT).[6] Patients who develop BOS post lung transplant vary in disease latency and severity.[6] Patients often initially have normal lung function on pulmonary function testing and have normal chest radiographs.[6] As the disease progresses they begin to have symptoms of shortness of breath, cough, and wheezing as their lung function declines. The Journal of Heart and Lung Transplantation published updated guidelines in 2001 for grading the severity of BOS.[20] The original guidelines and classification system were published in 1993 by the International Society for Heart and Lung Transplantation.[20] Their scoring system is based on the changes in FEV1 in patients from their baseline.[20] When patients are first diagnosed with BOS they have their baseline lung function established by doing pulmonary function testing at the time of diagnosis.[20] The BOS scoring system is as follows:
BOS 0: FEV1 > 90% of baseline and FEF25-75 > 75% of baseline
BOS 0-p: FEV1 81-89% of baseline and/or FEF25-75 <= 75% of baseline
BOS 1: FEV1 66-80% of baseline
BOS 2: FEV1 51-65% of baseline
BOS 3: FEV1 50% or less of baseline
The scoring system shows an increased severity of the disease as the BOS number increases.[20]
Hematopoietic stem cell transplant
Bronchiolitis obliterans affects up to 5.5% of people who have received HSCT.[21] One of the biggest risk factors after HSCT is the development of GVHD with a 14% risk.[22] Other risk factors post transplant including tobacco use, age of donor, age of recipient, lower baseline FEV1/FVC ratio, non-caucasian race, peripheral and lower circulating IgG levels.[6] Studies have, however, shown mixed results regarding these other risk factors. There has been an association shown between the increased use of peripheral stem cells and the risk of developing bronchiolitis obliterans.[6] Also, research has shown an increased risk for developing the disease within the first year of transplant if the person is infected with respiratory syncytial virus or parainfluenza virus within the first 100 days post transplant.[6]
Inhalants
There are many industrial inhalants that are known to cause various types of bronchiolitis, including bronchiolitis obliterans.[23]
Industrial workers who have presented with bronchiolitis:
- nylon-flock workers[19]
- workers who spray prints onto textiles with polyamide-amine dyes[19]
- battery workers who are exposed to thionyl chloride fumes
- workers at plants that use or manufacture flavorings such as diacetyl[9][19][24]
Diacetyl is a chemical used to produce the artificial butter flavoring[25] in many foods such as candy and microwave popcorn and occurring naturally in wines. This first came to public attention when eight former employees of the Gilster-Mary Lee popcorn plant in Jasper, Missouri developed bronchiolitis obliterans. Due to this event, bronchiolitis obliterans began to be referred to in the popular media as "popcorn lung" or "popcorn workers lung".[26][27][28][29] It is also referred to as "flavorings-related lung disease".[30]
Post-infectious
File:10.1371-journal.pone.0098381.g001.tif Typically found in young children and is the most common cause at this age.[31] Generally occurs after a viral infection of adenovirus (types 3, 7, and 21), measles (rubeola), mycoplasma, CMV, influenza, and parainfluenza.[4][6] Swyer-James syndrome is a rare complication of bronchiolitis obliterans caused by measles or adenovirus.[32] Post-infectious bronchiolitis obliterans is most common in the southern hemisphere particularly in countries such as Brazil, Argentina, Australia, Chile and New Zealand.[33] There was a large prevalence of the disease in these areas during the 1990s and early 2000s. In one hospital in Buenos Aires, the Ricardo Gutiérrez Children's hospital, the disease accounted for 14% of their inpatient respiratory population from 1993 to 2002.[33] As such, much of the information about post-infectious bronchiolitis obliterans has come from research out of South America. The most significant risk factors for the disease are infection with adenovirus and the need for ventilator support.[33] In contrast with another cause of bronchiolitis obliterans in children, Steven's Johnson's syndrome, post-infectious bronchiolitis obliterans tends to be a chronic but non-progressive disease.[31] The disease can have varying impact on children and their quality of life, which has been studied by lung function tests, as well as their exercise tolerance.[34] Children with lower lung function based on their pulmonary function testing, have lower exercise tolerance, which compounds the impact of the disease on cardiovascular function as they are not able to maintain age appropriate aerobic fitness.[34] This ultimately affects their activities of daily living (ADLs) and their quality of life going forward.[34]
Burn pits
A form of constrictive bronchiolitis is starting to present in Iraq and Afghanistan veterans. It has been attributed to veterans being exposed to trash burn pits. Veterans present with shortness of breath and other asthma-like symptoms. The only way to diagnose this condition is by doing a lung biopsy as chest X-rays and CT scans come back as normal. The US government still denies that there is any correlation between burn pits and health problems, but has started an "Airborne Hazards and Open Burn Pit Registry" to begin tracking the health of veterans who were exposed to burn pits to see if there is a connection.[35][36]
E-cigarettes
The American Lung Association lists flavored e-cigarettes as a risk in 2016.[37] Health Canada has, however, seen no cases as of 2019.[38] Public Health England writes that the association has come about as "some flavourings used in e-liquids to provide a buttery flavour contain the chemical diacetyl... however, diacetyl is banned as an ingredient from e-cigarettes and e-liquids in the UK."[39] The UK National Health Service's website states that "vaping does not cause popcorn lung".[40]
Mechanism
The underlying mechanism involves injury and inflammation of epithelial and sub-epithelial cells. These cells then lose the ability to repair the tissue, in particular they lose the ability to regenerate the epithelial or outermost layer, leading to the excess growth of cells that cause scarring.[11][6][1] There are multiple pathways of the disease including fibrotic, lymphocytic, and antibody-mediated that have been described. However, while each pathway has a more unique starting point and cause, the result is still injury and inflammation leading to scarring of the lung tissue.[11] The scarred tissue then makes the expiration phase of respiration more difficult, leading to air not being expelled from the lungs. This is termed "air-trapping", which can be seen on medical imaging.[6] Since the scarring is non-reversible, the disease generally does not improve over time, and depending on the inciting can progress to death.[11]
Diagnosis
Bronchiolitis obliterans is often diagnosed based on the symptoms of obstructive lung disease following lung injury. The definitive diagnosis is through biopsy, but due to the variable distribution of lesions, leading to falsely negative tests, and invasive nature of this procedure it is often not performed.[6][11] Several tests are often needed to diagnose bronchiolitis obliterans, including spirometry, diffusing capacity of the lung tests (DLCO), lung volume tests, chest X-rays, high-resolution CT (HRCT), and lung biopsy.[11][4]
Pulmonary function testing
Spirometry tests usually show an obstructive pattern and is the most common presentation.[6] A slightly reduced to normal forced vital capacity (FVC), and a reduced FEV1 to FVC ratio and forced expiratory volume (FEV) with little to no correction with the use of bronchodilators are common findings.[11][4] Lung volume tests may show hyperinflation (excessive air in lungs caused by air trapping). Diffusing capacity of the lung (DLCO) tests are usually normal; people with early-stage OB are more likely to have normal DLCO.[41] FEV1 (forced expiratory volume in 1 second) should be above 80% of predicted values to be considered normal. Bronchiolitis obliterans reduces this to between 16% and 21%.[42]
Medical imaging
Early in the disease chest radiography is typically normal but may show hyperinflation.[6] As the disease progresses a reticular pattern with thickening of airway walls may be present.[4][6] HRCT can also show air trapping when the person being scanned breathes out completely; it can also show thickening in the airway and haziness in the lungs.[11] A common finding on HRCT is patchy areas of decreased lung density, signifying reduced vascular caliber and air trapping. This pattern is often described as a "mosaic pattern", and may indicate bronchiolitis obliterans.[6]
Lung biopsy
Transthoracic lung biopsies are preferable for diagnosis of constrictive BO compared to transbronchial biopsies; regardless of the type of biopsy, a diagnosis may only be achieved by examination of multiple samples.[30] Transthoracic biopsies are preferred over transbronchial due to the heterogeneity and distribution of the lesions.[11] OB can be further classified into two categories: constrictive or proliferative.[11] The constrictive pattern is demonstrated by peribronchiolar cellular infiltrates which eventually causes small airway damage and leads to subepithelial fibrosis.[11] The bronchial muscle can eventually become fibrosed which can be identified with trichrome staining.[11] In regards to proliferative disease, intraluminal buds called "Masson bodies" fill the lumen, which results in bronchiolar plugging.[11] Often people with proliferative disease will show butterfly wing-like appearance under microscopy.[11] One key determinate that can be seen on biopsy to differentiate constrictive from proliferative disease is the extent of lesions. Both lesions are localized from the small bronchi to the membranous bronchi, but in constrictive disease, the lesions are intermittent while proliferative disease has a continuous distribution.[11]
Differential diagnosis
Other conditions that can present similarly include chronic obstructive pulmonary disease, asthma, bronchiectasis, hypersensitivity pneumonitis, and pneumonia.[30][43]
Prevention
Inhalants
Disease caused by exposure to industrial inhalants and burn pits can be prevented with the use of engineering controls (e.g., exhaust hoods or closed systems), personal protective equipment, monitoring of potentially affected personnel, worker education and training.[citation needed]
Transplant
The primary prevention of bronchiolitis obliterans in people who have received either lung transplant or HSCT therapy is immunosuppression.[6] In regards to post lung transplantation, the combination of calcineurin inhibitor combined with a purine synthesis inhibitor and a glucocorticoid is the general regimen used.[6] People also have a baseline post-transplant lung function testing done in order to determine if their lung function is declining over time. People who are post-HSCT their immunosuppressive regimen typically includes methotrexate in combination with a calcineurin inhibitor to prevent GVHD, a risk factor for developing bronchiolitis obliterans.[6]
Treatment
While the disease is not reversible, treatments can slow further worsening.[1] This may include the use of corticosteroids or immunosuppressive medication which may have an effect on the ability to receive a lung transplant if offered.[1][4] If patients have difficulty breathing (hypoxemia) oxygen can be supplemented. Routine vaccinations are recommended for patients with chronic lung disease to prevent complications from secondary infections due to pneumonia and influenza.[11]
Transplant recipients are at risk for re-developing the disease, as bronchiolitis obliterans is a form of chronic rejection. Evaluation of interventions for its prevention relies on early detection of abnormal spirometry results or unusual decreases in repeated measurements.[citation needed]
Terminology
"Bronchiolitis obliterans" was originally a term used by pathologists to describe two patterns of airway disease,[6] the other was bronchiolitis obliterans organizing pneumonia (BOOP), now known as cryptogenic organizing pneumonia.[6] The name cryptogenic bronchiolitis obliterans is used when a cause is unknown.[4]
Bronchiolitis obliterans when it occurs following a lung transplant is known as bronchiolitis obliterans syndrome (BOS).[11][4] BOS is defined as a person who has had either a HSCT or lung transplant and develops symptoms or radiographic findings consistent with bronchiolitis obliterans, but has not been confirmed by biopsy.[22][44]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 "Bronchiolitis obliterans". 2012. https://rarediseases.info.nih.gov/diseases/9551/bronchiolitis-obliterans.
- ↑ "Constrictive (obliterative) bronchiolitis: diagnosis, etiology, and a critical review of the literature". Annals of Diagnostic Pathology 2 (5): 321–34. October 1998. doi:10.1016/S1092-9134(98)80026-9. PMID 9845757.
- ↑ "Ventilation/perfusion scintigraphy in children with post-infectious bronchiolitis obliterans: a pilot study". PLOS ONE 9 (5): e98381. 2014. doi:10.1371/journal.pone.0098381. PMID 24852165. Bibcode: 2014PLoSO...998381X.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 4.18 4.19 "Obliterative (constrictive) bronchiolitis". Seminars in Respiratory and Critical Care Medicine 33 (5): 509–32. October 2012. doi:10.1055/s-0032-1325161. PMID 23001805.
- ↑ Lockey, Richard F.; Ledford, Dennis K. (2014). Asthma: Comorbidities, Coexisting Conditions, and Differential Diagnosis. Oxford University Press. p. 111. ISBN 9780199918072. https://books.google.com/books?id=9Do_AwAAQBAJ&pg=PA110.
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 6.12 6.13 6.14 6.15 6.16 6.17 6.18 6.19 6.20 6.21 6.22 "Obliterative bronchiolitis". The New England Journal of Medicine 370 (19): 1820–8. May 2014. doi:10.1056/NEJMra1204664. PMID 24806161.
- ↑ "Bronchiolitis obliterans. Roentgenologic-pathologic correlation". The American Journal of Roentgenology, Radium Therapy, and Nuclear Medicine 117 (4): 816–32. April 1973. doi:10.2214/ajr.117.4.816. PMID 4698820.
- ↑ Gourtsoyiannis, Nicholas C.; Ros, Pablo R. (2005). Radiologic-Pathologic Correlations from Head to Toe: Understanding the Manifestations of Disease. Springer Science & Business Media. p. 154. ISBN 9783540266648. https://books.google.com/books?id=7jNRJjptq-wC&pg=PA154.
- ↑ 9.0 9.1 Centers for Disease Control and Prevention (2002). Fixed obstructive lung disease in workers at a microwave popcorn factory (7th ed.).
- ↑ "More than a cold". https://www.morethanacold.co.uk/about-bronchiolitis/symptoms-to-watch-out-for/.
- ↑ 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 11.10 11.11 11.12 11.13 11.14 11.15 11.16 "Obliterative Bronchiolitis". Transplantation 100 (2): 272–83. February 2016. doi:10.1097/TP.0000000000000892. PMID 26335918.
- ↑ "Constrictive Bronchiolitis Attributable to Inhalation of Toxic Agents: Considerations for a Case Definition". Journal of Occupational and Environmental Medicine 60 (1): 90–96. January 2018. doi:10.1097/JOM.0000000000001176. PMID 28953074. http://insights.ovid.com/crossref?an=00043764-201801000-00014.
- ↑ "Sporadic Obliterative Bronchiolitis: Case Series and Systematic Review of the Literature". Mayo Clinic Proceedings. Innovations, Quality & Outcomes 3 (1): 86–93. March 2019. doi:10.1016/j.mayocpiqo.2018.10.003. PMID 30899912.
- ↑ Sam, Amir H.; Teo, James T.H. (2010). Rapid Medicine. Wiley-Blackwell. ISBN 978-1-4051-8323-9.
- ↑ "Activated charcoal bronchial aspiration". Jornal Brasileiro de Pneumologia 38 (4): 533–4. 2012. doi:10.1590/S1806-37132012000400018. PMID 22964940.
- ↑ "Sauropus androgynus (L.) Merr. Induced Bronchiolitis Obliterans: From Botanical Studies to Toxicology". Evidence-Based Complementary and Alternative Medicine 2015: 714158. 2015. doi:10.1155/2015/714158. PMID 26413127.
- ↑ Brant & Helms (1999). Fundamentals of Diagnostic Radiology. Baltimore: Williams & Wilkins. ISBN 978-0-683-30093-2.
- ↑ Webb (2000). High Resolution CT of the Lung (3rd ed.). Philadelphia: Lippincott Williams & Wilkins. ISBN 978-0-7817-0217-1. https://archive.org/details/highresolutionct0000webb_g7x6.
- ↑ 19.0 19.1 19.2 19.3 Brown, Jay A.. "Haz-Map; Information on Hazardous Chemicals and Occupational Diseases". National Institutes of Health. http://hazmap.nlm.nih.gov/cgi-bin/hazmap_generic?tbl=TblDiseases&id=551.
- ↑ 20.0 20.1 20.2 20.3 20.4 "Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria" (in en). The Journal of Heart and Lung Transplantation 21 (3): 297–310. March 2002. doi:10.1016/S1053-2498(02)00398-4. PMID 11897517. https://www.jhltonline.org/article/S1053-2498(02)00398-4/abstract.
- ↑ "Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation" (in en). Biology of Blood and Marrow Transplantation 17 (7): 1072–8. July 2011. doi:10.1016/j.bbmt.2010.11.018. PMID 21126596. PMC 3061253. https://www.bbmt.org/article/S1083-8791(10)00517-3/abstract.
- ↑ 22.0 22.1 "Azithromycin for the Treatment of Obliterative Bronchiolitis after Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis". Biology of Blood and Marrow Transplantation 22 (12): 2264–2269. December 2016. doi:10.1016/j.bbmt.2016.08.027. PMID 27575542.
- ↑ "Bronchiolitis. Pathologic considerations". American Journal of Clinical Pathology 109 (1): 101–9. January 1998. doi:10.1093/ajcp/109.1.101. PMID 9426525.
- ↑ California Department of Public Health
- ↑ "Diacetyl-induced lung disease". Toxicological Reviews 25 (4): 261–72. 2006. doi:10.2165/00139709-200625040-00006. PMID 17288497.
- ↑ Preventing lung disease in workers who make or use flavorings. National Institute for Occupational Safety and Health. 2004. doi:10.26616/NIOSHPUB2004110. https://www.cdc.gov/niosh/docs/2004-110/.
- ↑ "Popcorn worker's lung". The New England Journal of Medicine 347 (5): 360–1. August 2002. doi:10.1056/nejme020064. PMID 12151475.
- ↑ Egilman, David (2007). "Popcorn Workers Lung". http://www.ijoeh.com/pfds/IJOEH_1301_Egilman.pdf.
- ↑ "Newly recognized occupational and environmental causes of chronic terminal airways and parenchymal lung disease". Clinics in Chest Medicine 33 (4): 667–80. December 2012. doi:10.1016/j.ccm.2012.09.002. PMID 23153608.
- ↑ 30.0 30.1 30.2 "CDC - Flavorings-Related Lung Disease - NIOSH Workplace Safety and Health Topic". https://www.cdc.gov/niosh/topics/flavorings/.
- ↑ 31.0 31.1 "Post-infectious bronchiolitis obliterans in children". Jornal de Pediatria 87 (3): 187–98. 2011-05-05. doi:10.2223/JPED.2083. PMID 21547332.
- ↑ "Swyer-James Syndrome". American Journal of Respiratory and Critical Care Medicine 197 (1): 130–131. January 2018. doi:10.1164/rccm.201708-1691IM. PMID 29064268.
- ↑ 33.0 33.1 33.2 "Risk factors for the development of bronchiolitis obliterans in children with bronchiolitis". Thorax 61 (6): 503–6. June 2006. doi:10.1136/thx.2005.044909. PMID 16517579.
- ↑ 34.0 34.1 34.2 "Exercise Capacity in Children and Adolescents With Post-Infectious Bronchiolitis Obliterans: A Systematic Review". Revista Paulista de Pediatria 37 (2): 234–240. April 2019. doi:10.1590/1984-0462/;2019;37;2;00017. PMID 30892545.
- ↑ "Where There's Smoke: DoD Investigates Causes of Deployment-Related Pulmonary Symptoms Reported by Troops". http://www.usmedicine.com/compendium/where-theres-smoke-dod-investigates-causes-of-deployment-related-pulmonary-symptoms-reported-by-troops.html.
- ↑ Harrison Jacobs (5 November 2013). "Open-Air Burn Pits Leave Troops Sickly – Business Insider". Business Insider. http://www.businessinsider.com/open-air-burn-pits-leave-troops-sickly-2013-11.
- ↑ Editorial Staff. "Popcorn Lung: A Dangerous Risk of Flavored E-Cigarettes" (in en). https://www.lung.org/about-us/blog/2016/07/popcorn-lung-risk-ecigs.html.
- ↑ Canada, Health (31 July 2019). "Risks of vaping". https://www.canada.ca/en/health-canada/services/smoking-tobacco/vaping/risks.html#a4.
- ↑ "Clearing up some myths around e-cigarettes - Public health matters" (in en). 20 February 2018. https://publichealthmatters.blog.gov.uk/2018/02/20/clearing-up-some-myths-around-e-cigarettes/.
- ↑ "Vaping to quit smoking - Better Health" (in en). 2022-09-20. https://www.nhs.uk/better-health/quit-smoking/vaping-to-quit-smoking/.
- ↑ "Bronchiolitis Obliterans". https://www.lecturio.com/concepts/bronchiolitis-obliterans/.
- ↑ Krishna, R.; Anjum, F.; Oliver, T. I. (2022). Bronchiolitis Obliterans. PMID 28722895. https://www.ncbi.nlm.nih.gov/books/NBK441865/. Retrieved 5 July 2021.
- ↑ "Air trapping on expiratory high-resolution CT scans in the absence of inspiratory scan abnormalities: correlation with pulmonary function tests and differential diagnosis". AJR. American Journal of Roentgenology 170 (5): 1349–53. May 1998. doi:10.2214/ajr.170.5.9574614. PMID 9574614.
- ↑ "Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria" (in en). The Journal of Heart and Lung Transplantation 21 (3): 297–310. March 2002. doi:10.1016/S1053-2498(02)00398-4. PMID 11897517. https://www.jhltonline.org/article/S1053-2498(02)00398-4/abstract.
Further reading
- "Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan". The New England Journal of Medicine 365 (3): 222–30. July 2011. doi:10.1056/NEJMoa1101388. PMID 21774710.
- Brown JA. "Bronchiolitis obliterans". Haz-Map Information on Hazardous Chemicals and Occupational Diseases. National Institutes of Health. http://hazmap.nlm.nih.gov/cgi-bin/hazmap_generic?tbl=TblDiseases&id=551.
- (NIOSH) Alert: Preventing lung disease in workers who make or use flavorings. National Institute for Occupational Safety and Health. 2004. doi:10.26616/NIOSHPUB2004110. https://www.cdc.gov/niosh/docs/2004-110/.
- "Flavorings-Related Lung Disease". National Institute for Occupational Safety and Health. 2018-11-21. https://www.cdc.gov/niosh/topics/flavorings/.
External links
| Classification |
|---|
 |



