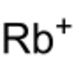Chemistry:Rubidium oxalate
| |||
|
| |||
| Identifiers | |||
|---|---|---|---|
| |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| Rb 2C 2O 4 | |||
| Molar mass | 258.954 g·mol−1 | ||
| Appearance | colourless crystals | ||
| Density | 2.76 g/cm3 (monohydrate) | ||
| Related compounds | |||
Other anions
|
|||
Other cations
|
| ||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
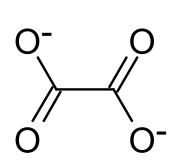
Rubidium oxalate is a chemical compound with the chemical formula Rb
2C
2O
4. It is a rubidium salt of oxalic acid. It consists of rubidium cations Rb+
and oxalate anions C
2O2−
4. Rubidium oxalate forms a monohydrate Rb
2C
2O
4 · H2O.
Preparation
Rubidium carbonate and oxalic acid react to form rubidium oxalate:[1]
- Rb
2CO
3 + H
2C
2O
4 → Rb
2C
2O
4 + H
2O + CO
2↑
Rubidium oxalate can also be obtained via the thermal decomposition of rubidium formate:[2]
- 2 HCOORb → Rb
2C
2O
4 + H
2↑
Properties
From an aqueous solution, rubidium oxalate crystallizes as a monohydrate Rb
2C
2O
4 · H2O in the monoclinic crystal system.[3] and is isomorphic to potassium oxalate monohydrate K
2C
2O
4 · H2O.[4] Two forms of the anhydrous form (Rb
2C
2O
4) exist at room temperature: one form is monoclinic and isotypic to caesium oxalate (Cs
2C
2O
4), the other is orthorhombic and isotypic to potassium oxalate (K
2C
2O
4).[5] Freshly prepared anhydrous rubidium oxalate initially contains mainly the monoclinic form, but this slowly transforms irreversibly into the orthorhombic form.[6] In 2004, two more high-temperature forms of rubidium oxalate were discovered.[7]
Crystal data of the different forms of rubidium oxalate
| Form | Crystal system | Space group | a in Å | b in Å | c in Å | β | Z |
|---|---|---|---|---|---|---|---|
| Alpha[5] | monoclinic | P21/c | 6.328 | 10.455 | 8.217 | 98.016° | 4 |
| Beta[5] | orthorhombic | Pbam | 11.288 | 6.295 | 3.622 | — | 2 |
| Monohydrate[8] | monoclinic | C2/c | 9.617 | 6.353 | 11.010 | 109.46° | 4 |
The standard enthalpy of formation of the crystalline rubidium oxalate is 1325.0 ± 8.1 kJ/mol.[9]
The decomposition of rubidium oxalate with the release of carbon monoxide and subsequently carbon dioxide and oxygen takes place at 507–527 °C (945–981 °F; 780–800 K).[6][2]
- Rb
2C
2O
4 → Rb
2CO
3 + CO↑
- Rb
2CO
3 → Rb
2O + CO
2↑
- 2 Rb
2O → 4 Rb + O
2↑
In addition to the neutral rubidium oxalate Rb
2C
2O
4, there is also an acidic salt, rubidium hydrogen oxalate with the formula RbHC
2O
4, which is isomorphic to potassium hydrogen oxalate KHC
2O
4[10] and forms monoclinic crystals,[11] and an acidic dioxalate with the formula RbHC
2O
4 · H
2C
2O
4, which exists as a dihydrate, has a density of 2.125 g/cm3 at 18 °C and a solubility of 21 g/L at 21 °C.[12]
Upon evaporation of a solution in hydrogen peroxide, rubidium oxalate forms a monoperhydrate of the formula Rb
2C
2O
4 · H2O
2, which forms monoclinic crystals that are relatively stable in air.[13]
Rubidium oxalate reacts with hydrogen fluoride to form a hydrofluoridate salt (RbHC
2O
4 · HF):[14]
- Rb
2C
2O
4 + 2 HF → RbHC
2O
4 · HF + RbF
References
- ↑ Giglio, E.; Loreti, S.; Pavel, N. V. (May 1988). "EXAFS: a new approach to the structure of micellar aggregates" (in en). The Journal of Physical Chemistry 92 (10): 2858–2862. doi:10.1021/j100321a032. ISSN 0022-3654. https://pubs.acs.org/doi/abs/10.1021/j100321a032.
- ↑ 2.0 2.1 Meisel, T.; Halmos, Z.; Seybold, K.; Pungor, E. (February 1975). "The thermal decomposition of alkali metal formates" (in en). Journal of Thermal Analysis 7 (1): 73–80. doi:10.1007/BF01911627. ISSN 0022-5215. http://link.springer.com/10.1007/BF01911627.
- ↑ Ans, Jean d'; Lax, Ellen (1998) (in de). Taschenbuch für Chemiker und Physiker. Springer. ISBN 978-3-540-60035-0. https://books.google.com/books?id=oWjEKDnsJgEC&pg=PA686.
- ↑ Pedersen, B. (1966-03-01). "The equilibrium hydrogen–hydrogen distances in the water molecules in potassium and rubidium oxalate monohydrates". Acta Crystallographica 20 (3): 412–417. doi:10.1107/S0365110X66000951. ISSN 0365-110X. https://scripts.iucr.org/cgi-bin/paper?S0365110X66000951.
- ↑ 5.0 5.1 5.2 Dinnebier, Robert E.; Vensky, Sascha; Panthöfer, Martin; Jansen, Martin (2003-03-10). "Crystal and molecular structures of alkali oxalates: first proof of a staggered oxalate anion in the solid state". Inorganic Chemistry 42 (5): 1499–1507. doi:10.1021/ic0205536. ISSN 0020-1669. PMID 12611516. https://pubmed.ncbi.nlm.nih.gov/12611516.
- ↑ 6.0 6.1 Vensky, Sascha (2004). Konformationsaufklärung anorganischer Oxoanionen des Kohlenstoffs und Festkörpersynthesen durch Elektrokristallisation von Ag3O4 und Na3BiO4 (doctoralThesis thesis) (in Deutsch).
- ↑ Robert E. Dinnebier, Sascha Vensky, Martin Jansen, Jonathan C. Hanson (2005-02-04), "Crystal Structures and Topological Aspects of the High-Temperature Phases and Decomposition Products of the Alkali-Metal Oxalates M2[C2O4] (M=K, Rb, Cs)", Chemistry - A European Journal 11 (4): 1119–1129, doi:10.1002/chem.200400616, PMID 15624128
- ↑ Takuya Echigo, Mitsuyoshi Kimata (November 2006), "The common role of water molecule and lone electron pair as a bond-valence mediator in oxalate complexes : the crystal structures of Rb2(C2O4) · H2O and Tl2(C2O4)", Zeitschrift für Kristallographie 221 (12): 762–769, doi:10.1524/zkri.2006.221.12.762, Bibcode: 2006ZK....221..762E
- ↑ Masuda, Y.; Miyamoto, H.; Kaneko, Y.; Hirosawa, K. (February 1985). "The standard molar enthalpies of formation of crystalline rubidium and cesium oxalates" (in en). The Journal of Chemical Thermodynamics 17 (2): 159–164. doi:10.1016/0021-9614(85)90068-0. https://linkinghub.elsevier.com/retrieve/pii/0021961485900680.
- ↑ Piccard, Julius (1862). "Beitrag zur Kenntniss der Rubidiumverbindungen" (in de). Journal für Praktische Chemie 86 (1): 449–460. doi:10.1002/prac.18620860163. ISSN 0021-8383. https://onlinelibrary.wiley.com/doi/10.1002/prac.18620860163.
- ↑ Watts, Henry (1866) (in en). A Dictionary of Chemistry and the Allied Branches of Other Sciences. Longmans, Green, Longman, Roberts & Green. https://books.google.com/books?id=va0EAAAAYAAJ&pg=PA264.
- ↑ Abegg, Richard Wilhelm Heinrich; Auerbach, Friedrich; Koppel, Ivan (1905). Handbuch der anorganischen Chemie. University of California. Leipzig, S. Hirzel. http://archive.org/details/handbuchderanor09koppgoog.
- ↑ Pedersen, Berit F.; Seip, Hans M.; Santesson, Johan; Holmberg, Pär; Eriksson, G.; Blinc, R.; Paušak, S.; Ehrenberg, L. et al. (1967). "The Crystal Structure of Potassium and Rubidium Oxalate Monoperhydrates, K2C2O4.H2O2 and Rb2C2O4.H2O2." (in en). Acta Chemica Scandinavica 21: 779–790. doi:10.3891/acta.chem.scand.21-0779. ISSN 0904-213X. http://actachemscand.org/doi/10.3891/acta.chem.scand.21-0779.
- ↑ Weinland, R. F.; Stille, W. (1903). "Ueber die Anlagerung von Krystallfluorwasserstoff an Oxalate und an Ammoniumtartrat" (in de). Justus Liebig's Annalen der Chemie 328 (2): 149–153. doi:10.1002/jlac.19033280205. https://onlinelibrary.wiley.com/doi/10.1002/jlac.19033280205.
 |