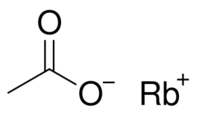
Chemistry:Rubidium acetate
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Rubidium acetate
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Molar mass | 144.51 g/mol |
| Appearance | White solid |
| Melting point | 246 °C (475 °F; 519 K) (decomposes) |
| 85 g/100 ml (45 °C)[2] | |
| log P | -0.561 |
| Hazards | |
| H305, H315 | |
| NFPA 704 (fire diamond) | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1 mg/m3 |
| Related compounds | |
Other anions
|
rubidium formate |
Other cations
|
Hydrogen acetate Lithium acetate Sodium acetate Potassium acetate Caesium acetate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Rubidium acetate is a rubidium salt that is the result of reacting rubidium metal, rubidium carbonate, or rubidium hydroxide with acetic acid. It is soluble in water like other acetates.[2]
Uses
Rubidium acetate is used as a catalyst for the polymerization of silanol terminated siloxane oligomers.[5]
References
- ↑ "Rubidium acetate". https://pubchem.ncbi.nlm.nih.gov/compound/23673628.
- ↑ 2.0 2.1 2.2 "CXRB010_ RUBIDIUM ACETATE, monohydrate" (PDF). http://www.gelest.com/wp-content/uploads/product_msds/CXRB010-msds.pdf. Retrieved 2021-02-03.
- ↑ "RUBIDIUM ACETATE | 563-67-7". https://www.chemicalbook.com/ChemicalProductProperty_EN_CB1126064.htm.
- ↑ "Safety data sheet". s3.amazonaws.com. 2015. https://s3.amazonaws.com/gelest/sds/CXRB010_GHS+US_English+US.pdf. Retrieved 2021-02-03.
- ↑ "Rubidium acetate". https://www.gelest.com/product/CXRB010/.
Acetyl halides and salts of the acetate ion
| |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AcOH | He | ||||||||||||||||||
| LiOAc | Be(OAc)2 BeAcOH |
B(OAc)3 | AcOAc ROAc |
NH4OAc | AcOOH | FAc | Ne | ||||||||||||
| NaOAc | Mg(OAc)2 | Al(OAc)3 ALSOL Al(OAc)2OH Al2SO4(OAc)4 |
Si | P | S | ClAc | Ar | ||||||||||||
| KOAc | Ca(OAc)2 | Sc(OAc)3 | Ti(OAc)4 | VO(OAc)3 | Cr(OAc)2 Cr(OAc)3 |
Mn(OAc)2 Mn(OAc)3 |
Fe(OAc)2 Fe(OAc)3 |
Co(OAc)2, Co(OAc)3 |
Ni(OAc)2 | Cu(OAc)2 | Zn(OAc)2 | Ga(OAc)3 | Ge | As(OAc)3 | Se | BrAc | Kr | ||
| RbOAc | Sr(OAc)2 | Y(OAc)3 | Zr(OAc)4 | Nb | Mo(OAc)2 | Tc | Ru(OAc)2 Ru(OAc)3 Ru(OAc)4 |
Rh2(OAc)4 | Pd(OAc)2 | AgOAc | Cd(OAc)2 | In | Sn(OAc)2 Sn(OAc)4 |
Sb(OAc)3 | Te | IAc | Xe | ||
| CsOAc | Ba(OAc)2 | Hf | Ta | W | Re | Os | Ir | Pt(OAc)2 | Au | Hg2(OAc)2, Hg(OAc)2 |
TlOAc Tl(OAc)3 |
Pb(OAc)2 Pb(OAc)4 |
Bi(OAc)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La(OAc)3 | Ce(OAc)x | Pr | Nd | Pm | Sm(OAc)3 | Eu(OAc)3 | Gd(OAc)3 | Tb | Dy(OAc)3 | Ho(OAc)3 | Er | Tm | Yb(OAc)3 | Lu(OAc)3 | |||||
| Ac | Th | Pa | UO2(OAc)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
 |

