Chemistry:Zavegepant
From HandWiki
Short description: Medication for treatment of migraine
 | |
| Clinical data | |
|---|---|
| Trade names | Zavzpret |
| Other names | BHV-3500 |
| License data |
|
| Routes of administration | Nasal |
| Drug class | Calcitonin gene-related peptide receptor antagonist |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEMBL |
|
| Chemical and physical data | |
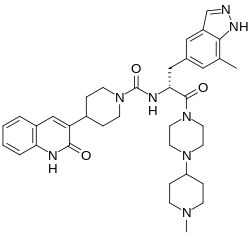
| Formula | C36H46N8O3 |
| Molar mass | 638.817 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Zavegepant, sold under the brand name Zavzpret, is a medication used for the treatment of migraine.[1] Zavegepant is a calcitonin gene-related peptide receptor antagonist.[1] It is sprayed into the nose.[1] It is sold by Pfizer.[1]
The most common adverse reactions include taste disorders, nausea, nasal discomfort, and vomiting.[1]
Zavegepant was approved for medical use in the United States in March 2023.[1][2][3][4]
Medical uses
Zavegepant is indicated for the acute treatment of migraine with or without aura in adults.[1][5][6]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Zavzpret- zavegepant spray". 9 March 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9c4a7aec-daef-4961-ba77-92f4b58be780.
- ↑ "Drug Approval Package: Zavzpret". 3 April 2023. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2023/216386Orig1s000TOC.cfm.
- ↑ "Pfizer's Zavzpret (Zavegepant) Migraine Nasal Spray Receives FDA Approval" (Press release). 10 March 2023.
- ↑ "Zavegepant: First Approval". Drugs 83 (9): 825–831. June 2023. doi:10.1007/s40265-023-01885-6. PMID 37227596.
- ↑ "Safety and Efficacy of Zavegepant in Treating Migraine: A Systematic Review". Cureus 15 (7): e41991. July 2023. doi:10.7759/cureus.41991. PMID 37593294.
- ↑ "Zavegepant Intranasal Spray for Migraines". The Annals of Pharmacotherapy: 10600280231209439. October 2023. doi:10.1177/10600280231209439. PMID 37897226.
Further reading
- "Zavegepant nasal spray for the acute treatment of migraine: A Phase 2/3 double-blind, randomized, placebo-controlled, dose-ranging trial". Headache 62 (9): 1153–1163. October 2022. doi:10.1111/head.14389. PMID 36239038.
- "A Comprehensive Review of Zavegepant as Abortive Treatment for Migraine". Health Psychology Research 10 (3): 35506. 2022. doi:10.52965/001c.35506. PMID 35774914.
- "Focus on zavegepant: the first intranasal third-generation gepant". Pain Management 12 (8): 879–885. November 2022. doi:10.2217/pmt-2022-0054. PMID 36189708.
External links
- Clinical trial number NCT04571060 for "Randomized Trial in Adult Subjects With Acute Migraines" at ClinicalTrials.gov
- Clinical trial number NCT03872453 for "Acute Treatment Trial in Adult Subjects With Migraines" at ClinicalTrials.gov
 |

