Biology:Xanthine oxidase
| xanthine oxidase/dehydrogenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
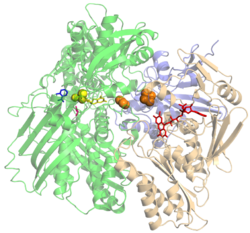 Crystallographic structure (monomer) of bovine xanthine oxidase.[1] The bounded FAD (red), FeS-cluster (orange), the molybdopterin cofactor with molybdenum (yellow) and salicylate (blue) are indicated. | |||||||||
| Identifiers | |||||||||
| EC number | 1.17.3.2 | ||||||||
| CAS number | 9002-17-9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| xanthine oxidase/dehydrogenase | |
|---|---|
| Identifiers | |
| Symbol | XDH |
| NCBI gene | 7498 |
| HGNC | 12805 |
| OMIM | 607633 |
| PDB | 1FIQ |
| RefSeq | NM_000379 |
| UniProt | P47989 |
| Other data | |
| EC number | 1.17.3.2 |
| Locus | Chr. 2 p23.1 |
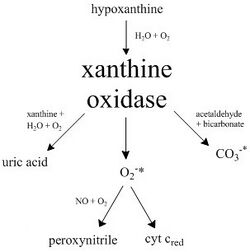
Xanthine oxidase (XO, sometimes 'XAO') is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species.[2] These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans.[3]
Xanthine oxidase is defined as an enzyme activity (EC 1.17.3.2).[4] The same protein, which in humans has the HGNC approved gene symbol XDH, can also have xanthine dehydrogenase activity (EC 1.17.1.4).[5] Most of the protein in the liver exists in a form with xanthine dehydrogenase activity, but it can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification.[6][7]
Reaction
The following chemical reactions are catalyzed by xanthine oxidase:
- hypoxanthine + H2O + O2 ⇌ xanthine + H2O2
- xanthine + H2O + O2 ⇌ uric acid + H2O2
- Xanthine oxidase can also act on certain other purines, pterins, and aldehydes. For example, it efficiently converts 1-methylxanthine (a metabolite of caffeine) to 1-methyluric acid, but has little activity on 3-methylxanthine.[8]
- Under some circumstances it can produce superoxide ions: RH + H2O + 2 O2 ⇌ ROH + 2 O−2 + 2 H+.[5]
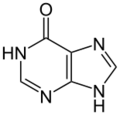
hypoxanthine (one oxygen atom)
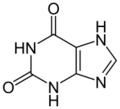
xanthine (two oxygens)
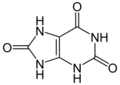
uric acid (three oxygens)
Other reactions
Because XO is a superoxide-producing enzyme, with general low specificity,[9] it can be combined with other compounds and enzymes and create reactive oxidants, as well as oxidize other substrates.
Bovine xanthine oxidase (from milk) was originally thought to have a binding site to reduce cytochrome c with, but it has been found that the mechanism to reduce this protein is through XO's superoxide anion byproduct, with competitive inhibition by carbonic anhydrase.[10]
Another reaction catalyzed by xanthine oxidase is the decomposition of S-nitrosothiols (RSNO), a class of reactive nitrogen species, to nitric oxide (NO), which reacts with a superoxide anion to form peroxynitrite under aerobic conditions.[11]
XO has also been found to produce the strong one-electron oxidant carbonate radical anion from oxidation with acetaldehyde in the presence of catalase and bicarbonate. It was suggested that the carbonate radical was likely produced in one of the enzyme's redox centers with a peroxymonocarbonate intermediate.[9]
Here is a diagram highlighting the pathways catalyzed by xanthine oxidase.
It is suggested that xanthine oxidoreductase, along with other enzymes, participates in the conversion of nitrate to nitrite in mammalian tissues.[12]
Protein structure
The protein is large, having a molecular weight of 270 kDa, and has two flavin molecules (bound as FAD), 2 molybdenum atoms, and 8 iron atoms bound per enzymatic unit. The molybdenum atoms are contained as molybdopterin cofactors and are the active sites of the enzyme. The iron atoms are part of [2Fe-2S] ferredoxin iron-sulfur clusters and participate in electron transfer reactions.
Catalytic mechanism
The active site of XO is composed of a molybdopterin unit with the molybdenum atom also coordinated by terminal oxygen (oxo), sulfur atoms and a terminal hydroxide. In the reaction with xanthine to form uric acid, an oxygen atom is transferred from molybdenum to xanthine, whereby several intermediates are assumed to be involved.[3] The reformation of the active molybdenum center occurs by the addition of water. Like other known molybdenum-containing oxidoreductases, the oxygen atom introduced to the substrate by XO originates from water rather than from dioxygen (O2).
Clinical significance
Xanthine oxidase is a superoxide-producing enzyme found normally in serum and the lungs, and its activity is increased during influenza A infection.[13]
During severe liver damage, xanthine oxidase is released into the blood, so a blood assay for XO is a way to determine if liver damage has happened.[14]
Because xanthine oxidase is a metabolic pathway for uric acid formation, the xanthine oxidase inhibitor allopurinol is used in the treatment of gout. Since xanthine oxidase is involved in the metabolism of 6-mercaptopurine, caution should be taken before administering allopurinol and 6-mercaptopurine, or its prodrug azathioprine, in conjunction.
Xanthinuria is a rare genetic disorder where the lack of xanthine oxidase leads to high concentration of xanthine in blood and can cause health problems such as renal failure. There is no specific treatment, affected people are advised by doctors to avoid foods high in purine and to maintain a high fluid intake. Type I xanthinuria has been traced directly to mutations of the XDH gene which mediates xanthine oxidase activity. Type II xanthinuria may result from a failure of the mechanism which inserts sulfur into the active sites of xanthine oxidase and aldehyde oxidase, a related enzyme with some overlapping activities (such as conversion of allopurinol to oxypurinol).[15]
Inhibition of xanthine oxidase has been proposed as a mechanism for improving cardiovascular health.[16] A study found that patients with chronic obstructive pulmonary disease (COPD) had a decrease in oxidative stress, including glutathione oxidation and lipid peroxidation, when xanthine oxidase was inhibited using allopurinol.[17] Oxidative stress can be caused by hydroxyl free radicals and hydrogen peroxide, both of which are byproducts of XO activity.[18]
Increased concentration of serum uric acid has been under research as an indicator for cardiovascular health factors, and has been used to strongly predict mortality, heart transplant, and more in patients.[16] But it is not clear whether this could be a direct or casual association or link between serum uric acid concentration (and by proxy, xanthine oxidase activity) and cardiovascular health.[19] States of high cell turnover and alcohol ingestion are some of the most prominent cases of high serum uric acid concentrations.[18]
Reactive nitrogen species, such as peroxynitrite that xanthine oxidase can form, have been found to react with DNA, proteins, and cells, causing cellular damage or even toxicity. Reactive nitrogen signaling, coupled with reactive oxygen species, have been found to be a central part of myocardial and vascular function, explaining why xanthine oxidase is being researched for links to cardiovascular health.[20]
Both xanthine oxidase and xanthine oxidoreductase are also present in corneal epithelium and endothelium and may be involved in oxidative eye injury.[21]
Inhibitors
Inhibitors of XO include allopurinol,[22] oxypurinol,[23] and phytic acid.[24] It has also been found to be inhibited by flavonoids,[25] including those found in Bougainvillea spectabilis (Nyctaginaceae) leaves (with an IC50 of 7.23 μM), typically used in folk medicine.[26]
See also
References
- ↑ PDB: 1FIQ; "Crystal structures of bovine milk xanthine dehydrogenase and xanthine oxidase: structure-based mechanism of conversion". Proceedings of the National Academy of Sciences of the United States of America 97 (20): 10723–8. September 2000. doi:10.1073/pnas.97.20.10723. PMID 11005854. Bibcode: 2000PNAS...9710723E.
- ↑ "Comparative histochemical and immunohistochemical study on xanthine oxidoreductase/xanthine oxidase in mammalian corneal epithelium". Acta Histochemica 106 (1): 69–75. February 2004. doi:10.1016/j.acthis.2003.08.001. PMID 15032331.
- ↑ 3.0 3.1 "The Mononuclear Molybdenum Enzymes". Chemical Reviews 114 (7): 3963–4038. April 2014. doi:10.1021/cr400443z. PMID 24467397.
- ↑ "KEGG record for EC 1.17.3.2". http://www.genome.jp/dbget-bin/www_bget?ec:1.17.3.2.
- ↑ 5.0 5.1 "KEGG record for EC 1.17.1.4". http://www.genome.jp/dbget-bin/www_bget?ec:1.17.1.4.
- ↑ "Entrez Gene: XDH xanthine dehydrogenase". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=7498.
- ↑ Online Mendelian Inheritance in Man (OMIM) Xanthine dehydrogenase; XDH -607633
- ↑ "1-Methylxanthine derived from caffeine as a pharmacodynamic probe of oxypurinol effect". British Journal of Clinical Pharmacology 43 (2): 197–200. February 1997. doi:10.1046/j.1365-2125.1997.53711.x. PMID 9131954.
- ↑ 9.0 9.1 "Production of the carbonate radical anion during xanthine oxidase turnover in the presence of bicarbonate". The Journal of Biological Chemistry 279 (50): 51836–43. December 2004. doi:10.1074/jbc.M406929200. PMID 15448145.
- ↑ "The reduction of cytochrome c by milk xanthine oxidase". The Journal of Biological Chemistry 243 (21): 5753–60. November 1968. doi:10.1016/S0021-9258(18)91929-0. PMID 4972775. http://www.jbc.org/content/243/21/5753.short.
- ↑ "Xanthine oxidase-mediated decomposition of S-nitrosothiols". The Journal of Biological Chemistry 273 (14): 7828–34. April 1998. doi:10.1074/jbc.273.14.7828. PMID 9525875.
- ↑ Jansson, E. A.; Huang, L.; Malkey, R.; Govoni, M.; Nihlén, C.; Olsson, A.; Stensdotter, M.; Petersson, J. et al. (2008). "A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis". Nature Chemical Biology 4 (7): 411–7. doi:10.1038/nchembio.92. PMID 18516050.
- ↑ "Vitamin C and the common cold". The British Journal of Nutrition 67 (1): 3–16. January 1992. doi:10.1079/BJN19920004. PMID 1547201. http://www.colorado.edu/intphys/iphy3700/vitCHemila92.pdf. Retrieved 28 October 2011.
- ↑ "Serum xanthine oxidase in human liver disease". The American Journal of Gastroenterology 96 (4): 1194–9. April 2001. doi:10.1111/j.1572-0241.2001.03700.x. PMID 11316169.
- ↑ Online Mendelian Inheritance in Man (OMIM) Xanthinuria, Type II; XAN2 -603592
- ↑ 16.0 16.1 "Uric acid and xanthine oxidase: future therapeutic targets in the prevention of cardiovascular disease?". British Journal of Clinical Pharmacology 62 (6): 633–44. December 2006. doi:10.1111/j.1365-2125.2006.02785.x. PMID 21894646.
- ↑ "Xanthine oxidase is involved in exercise-induced oxidative stress in chronic obstructive pulmonary disease". The American Journal of Physiology 277 (6 Pt 2): R1697–704. December 1999. doi:10.1152/ajpregu.1999.277.6.R1697. PMID 10600916.
- ↑ 18.0 18.1 "The potential for xanthine oxidase inhibition in the prevention and treatment of cardiovascular and cerebrovascular disease". Cardiovascular Psychiatry and Neurology 2009: 1–9. 2009. doi:10.1155/2009/282059. PMID 20029618.
- ↑ "Uric acid reduction: a new paradigm in the management of cardiovascular risk?". Current Medicinal Chemistry 14 (17): 1879–86. 2007. doi:10.2174/092986707781058797. PMID 17627523.
- ↑ "Nitroso-redox interactions in the cardiovascular system". Circulation 114 (14): 1531–44. October 2006. doi:10.1161/CIRCULATIONAHA.105.605519. PMID 17015805.
- ↑ "Xanthine oxidoreductase and xanthine oxidase in human cornea". Histology and Histopathology 17 (3): 755–60. 2002. doi:10.14670/HH-17.755. PMID 12168784.
- ↑ "Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol". Pharmacological Reviews 58 (1): 87–114. March 2006. doi:10.1124/pr.58.1.6. PMID 16507884.
- ↑ "Oxypurinol as an inhibitor of xanthine oxidase-catalyzed production of superoxide radical". Biochemical Pharmacology 37 (2): 349–52. Jan 1988. doi:10.1016/0006-2952(88)90739-3. PMID 2829916.
- ↑ "Inhibition of xanthine oxidase by phytic acid and its antioxidative action". Life Sciences 74 (13): 1691–700. February 2004. doi:10.1016/j.lfs.2003.09.040. PMID 14738912.
- ↑ "Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers". Journal of Natural Products 61 (1): 71–6. Jan 1998. doi:10.1021/np970237h. PMID 9461655.
- ↑ "Inhibitory effects of flavonoids on xanthine oxidase". Anticancer Research 13 (6A): 2165–70. Nov–Dec 1993. PMID 8297130.
External links
- Xanthine+Oxidase at the US National Library of Medicine Medical Subject Headings (MeSH)
 |