Biology:Adenosine deaminase
 Generic protein structure example |
| Adenosine/AMP deaminase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
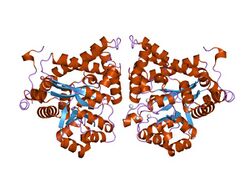 crystal structure of plasmodium yoelii adenosine deaminase (py02076) | |||||||||
| Identifiers | |||||||||
| Symbol | A_deaminase | ||||||||
| Pfam | PF00962 | ||||||||
| Pfam clan | CL0034 | ||||||||
| InterPro | IPR001365 | ||||||||
| PROSITE | PDOC00419 | ||||||||
| SCOP2 | 1add / SCOPe / SUPFAM | ||||||||
| CDD | cd01320 | ||||||||
| |||||||||
| Adenosine deaminase (editase) domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | A_deamin | ||||||||
| Pfam | PF02137 | ||||||||
| InterPro | IPR002466 | ||||||||
| PROSITE | PDOC00419 | ||||||||
| SCOP2 | 1add / SCOPe / SUPFAM | ||||||||
| |||||||||
| Adenosine/AMP deaminase N-terminal | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | A_deaminase_N | ||||||||
| Pfam | PF08451 | ||||||||
| InterPro | IPR013659 | ||||||||
| |||||||||
Adenosine deaminase (also known as adenosine aminohydrolase, or ADA) is an enzyme (EC 3.5.4.4) involved in purine metabolism. It is needed for the breakdown of adenosine from food and for the turnover of nucleic acids in tissues.
Its primary function in humans is the development and maintenance of the immune system.[1] However, the full physiological role of ADA is not yet completely understood.[2]
Structure
ADA exists in both small form (as a monomer) and large form (as a dimer-complex).[2] In the monomer form, the enzyme is a polypeptide chain,[3] folded into eight strands of parallel α/β barrels, which surround a central deep pocket that is the active site.[1] In addition to the eight central β-barrels and eight peripheral α-helices, ADA also contains five additional helices: residues 19-76 fold into three helices, located between β1 and α1 folds; and two antiparallel carboxy-terminal helices are located across the amino-terminal of the β-barrel.
The ADA active site contains a zinc ion, which is located in the deepest recess of the active site and coordinated by five atoms from His15, His17, His214, Asp295, and the substrate.[1] Zinc is the only cofactor necessary for activity.
The substrate, adenosine, is stabilized and bound to the active site by nine hydrogen bonds.[1] The carboxyl group of Glu217, roughly coplanar with the substrate purine ring, is in position to form a hydrogen bond with N1 of the substrate. The carboxyl group of Asp296, also coplanar with the substrate purine ring, forms hydrogen bond with N7 of the substrate. The NH group of Gly184 is in position to form a hydrogen bond with N3 of the substrate. Asp296 forms bonds both with the Zn2+ ion as well as with 6-OH of the substrate. His238 also hydrogen bonds to substrate 6-OH. The 3'-OH of the substrate ribose forms a hydrogen bond with Asp19, while the 5'-OH forms a hydrogen bond with His17. Two further hydrogen bonds are formed to water molecules, at the opening of the active site, by the 2'-OH and 3'-OH of the substrate.
Due to the recessing of the active site inside the enzyme, the substrate, once bound, is almost completely sequestered from solvent.[1] The surface exposure of the substrate to solvent when bound is 0.5% the surface exposure of the substrate in the free state.
Reactions
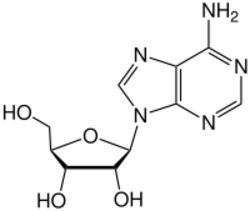
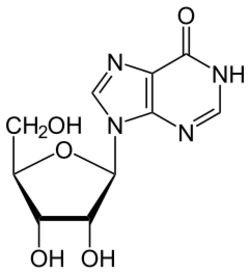
ADA irreversibly deaminates adenosine, converting it to the related nucleoside inosine by the substitution of the amino group by a keto group.
Inosine can then be deribosylated (removed from ribose) by another enzyme called purine nucleoside phosphorylase (PNP), converting it to hypoxanthine.
Mechanism of catalysis
The proposed mechanism for ADA-catalyzed deamination is stereospecific addition-elimination via tetrahedral intermediate.[4] By either mechanism, Zn2+ as a strong electrophile activates a water molecule, which is deprotonated by the basic Asp295 to form the attacking hydroxide.[1] His238 orients the water molecule and stabilizes the charge of the attacking hydroxide. Glu217 is protonated to donate a proton to N1 of the substrate.
The reaction is stereospecific due to the location of the zinc, Asp295, and His238 residues, which all face the B-side of the purine ring of the substrate.[1]
Competitive inhibition has been observed for ADA, where the product inosine acts at the competitive inhibitor to enzymatic activity.[5]
Function
ADA is considered one of the key enzymes of purine metabolism.[4] The enzyme has been found in bacteria, plants, invertebrates, vertebrates, and mammals, with high conservation of amino acid sequence.[2] The high degree of amino acid sequence conservation suggests the crucial nature of ADA in the purine salvage pathway.
Primarily, ADA in humans is involved in the development and maintenance of the immune system. However, ADA association has also been observed with epithelial cell differentiation, neurotransmission, and gestation maintenance.[6] It has also been proposed that ADA, in addition to adenosine breakdown, stimulates release of excitatory amino acids and is necessary to the coupling of A1 adenosine receptors and heterotrimeric G proteins.[2] Adenosine deaminase deficiency leads to pulmonary fibrosis,[7] suggesting that chronic exposure to high levels of adenosine can exacerbate inflammation responses rather than suppressing them. It has also been recognized that AMP deaminase protein and activity is upregulated in mouse hearts that overexpress HIF-1α,[8] which in part explains the attenuated levels of adenosine in HIF-1α expressing hearts during ischemic stress.[9]
In meiotic and post-meiotic male germ cells ADA2 regulates heterochromatin via translation of the MDC1 gene.[10]
Pathology
Some mutations in the gene for adenosine deaminase cause it not to be expressed. The resulting deficiency is one cause of severe combined immunodeficiency (SCID), particularly of autosomal recessive inheritance.[11] Deficient levels of ADA have also been associated with pulmonary inflammation, thymic cell death, and defective T-cell receptor signaling.[12][13]
Conversely, mutations causing this enzyme to be overexpressed are one cause of hemolytic anemia.[14]
There is some evidence that a different allele (ADA2) may lead to autism.[15]
Elevated levels of ADA has also been associated with AIDS.[12][16]
Isoforms
There are 2 isoforms of ADA: ADA1 and ADA2.
- ADA1 is found in most body cells, particularly lymphocytes and macrophages, where it is present not only in the cytosol and nucleus but also as the ecto- form on the cell membrane attached to dipeptidyl peptidase-4 (aka, CD26). ADA1 is involved mostly in intracellular activity, and exists both in small form (monomer) and large form (dimer).[2] The interconversion of small to large forms is regulated by a 'conversion factor' in the lung.[17]
- ADA2 was first identified in human spleen.[18] It was subsequently found in other tissues including the macrophage where it co-exists with ADA1. The two isoforms regulate the ratio of adenosine to deoxyadenosine potentiating the killing of parasites. ADA2 is found predominantly in the human plasma and serum, and exists solely as a homodimer.[19]
Clinical significance
ADA2 is the predominant form present in human blood plasma and is increased in many diseases, particularly those associated with the immune system: for example rheumatoid arthritis, psoriasis, and sarcoidosis. The plasma ADA2 isoform is also increased in most cancers. ADA2 is not ubiquitous but co-exists with ADA1 only in monocytes-macrophages.[citation needed]
Total plasma ADA can be measured using high performance liquid chromatography or enzymatic or colorimetric techniques. Perhaps the simplest system is the measurement of the ammonia released from adenosine when broken down to inosine. After incubation of plasma with a buffered solution of adenosine the ammonia is reacted with a Berthelot reagent to form a blue colour which is proportionate to the amount of enzyme activity. To measure ADA2, erythro-9-(2-hydroxy-3-nonyl) adenine (EHNA) is added prior to incubation so as to inhibit the enzymatic activity of ADA1.[18] It is the absence of ADA1 that causes SCID.
ADA can also be used in the workup of lymphocytic pleural effusions or peritoneal ascites, in that such specimens with low ADA levels essentially excludes tuberculosis from consideration.[20]
Tuberculosis pleural effusions can now be diagnosed accurately by increased levels of pleural fluid adenosine deaminase, above 40 U per liter.[21]
Cladribine and Pentostatin are anti-neoplastic agents used in the treatment of hairy cell leukemia; their mechanism of action is inhibition of adenosine deaminase.
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Atomic structure of adenosine deaminase complexed with a transition-state analog: understanding catalysis and immunodeficiency mutations". Science 252 (5010): 1278–1284. May 1991. doi:10.1126/science.1925539. PMID 1925539. Bibcode: 1991Sci...252.1278W.
- ↑ 2.0 2.1 2.2 2.3 2.4 "Adenosine deaminase: functional implications and different classes of inhibitors". Medicinal Research Reviews 21 (2): 105–128. Mar 2001. doi:10.1002/1098-1128(200103)21:2<105::AID-MED1002>3.0.CO;2-U. PMID 11223861.
- ↑ "Human adenosine deaminase. Purification and subunit structure". The Journal of Biological Chemistry 252 (1): 110–115. Jan 1977. doi:10.1016/S0021-9258(17)32805-3. PMID 13062.
- ↑ 4.0 4.1 "Crystal structure of Staphylococcus aureus tRNA adenosine deaminase TadA in complex with RNA". Nature Structural & Molecular Biology 13 (2): 153–159. Jan 2006. doi:10.1038/nsmb1047. PMID 16415880.
- ↑ "A product inhibition study on adenosine deaminase by spectroscopy and calorimetry". Journal of Biochemistry and Molecular Biology 35 (3): 302–305. May 2002. doi:10.5483/BMBRep.2002.35.3.302. PMID 12297022.
- ↑ "Enzymes involved in purine metabolism--a review of histochemical localization and functional implications". Histology and Histopathology 14 (4): 1321–1340. Oct 1999. PMID 10506947.
- ↑ "Too much of a good thing: adenosine overload in adenosine-deaminase-deficient mice". Trends in Pharmacological Sciences 24 (2): 66–70. 2003. doi:10.1016/S0165-6147(02)00045-7. PMID 12559769.
- ↑ Wu, Joe (2014). "4". HIF-1α in the Heart: Provision of Ischemic Cardioprotection and Remodeling of Nucleotide Metabolism (dissertation).
- ↑ "HIF-1α in the heart: remodeling nucleotide metabolism". Journal of Molecular and Cellular Cardiology 82: 194–200. 2015. doi:10.1016/j.yjmcc.2015.01.014. PMID 25681585.
- ↑ Chukrallah LG, Badrinath A, Vittor GG, Snyder EM. ADAD2 regulates heterochromatin in meiotic and post-meiotic male germ cells via translation of MDC1. J Cell Sci. 2022 Feb 15;135(4):jcs259196. doi: 10.1242/jcs.259196. Epub 2022 Feb 22. PMID 35191498; PMCID: PMC8919335
- ↑ "Carrier frequency of a nonsense mutation in the adenosine deaminase (ADA) gene implies a high incidence of ADA-deficient severe combined immunodeficiency (SCID) in Somalia and a single, common haplotype indicates common ancestry". Annals of Human Genetics 71 (Pt 3): 336–47. May 2007. doi:10.1111/j.1469-1809.2006.00338.x. PMID 17181544.
- ↑ 12.0 12.1 Adenosine Deaminase Deficiency: Metabolic Basis of Immune Deficiency and Pulmonary Inflammation. Advances in Immunology. 86. 2005. pp. 1–41. doi:10.1016/S0065-2776(04)86001-2. ISBN 9780120044863.
- ↑ "Adenosine deaminase deficiency increases thymic apoptosis and causes defective T cell receptor signaling". The Journal of Clinical Investigation 108 (1): 131–141. Jul 2001. doi:10.1172/JCI10360. PMID 11435465.
- ↑ "Elevated adenosine deaminase activity and hereditary hemolytic anemia. Evidence for abnormal translational control of protein synthesis". The Journal of Clinical Investigation 79 (3): 1001–5. Mar 1987. doi:10.1172/JCI112866. PMID 3029177.
- ↑ "Adenosine deaminase alleles and autistic disorder: case-control and family-based association studies". American Journal of Medical Genetics 96 (6): 784–90. Dec 2000. doi:10.1002/1096-8628(20001204)96:6<784::AID-AJMG18>3.0.CO;2-7. PMID 11121182.
- ↑ "Elevated erythrocyte adenosine deaminase activity in patients with acquired immunodeficiency syndrome". Proceedings of the National Academy of Sciences of the United States of America 83 (4): 1089–1091. Feb 1986. doi:10.1073/pnas.83.4.1089. PMID 3006027. Bibcode: 1986PNAS...83.1089C.
- ↑ "Purification and subunit structure of adenosine deaminase from human kidney". The Journal of Biological Chemistry 252 (18): 6409–6415. Sep 1977. doi:10.1016/S0021-9258(17)39973-8. PMID 893413.
- ↑ 18.0 18.1 "Characterization of the residual adenosine deaminating activity in the spleen of a patient with combined immunodeficiency disease and adenosine deaminase deficiency". Proceedings of the National Academy of Sciences of the United States of America 75 (1): 446–50. Jan 1978. doi:10.1073/pnas.75.1.446. PMID 24216. Bibcode: 1978PNAS...75..446S.
- ↑ "Human ADA2 belongs to a new family of growth factors with adenosine deaminase activity". The Biochemical Journal 391 (Pt 1): 51–57. Oct 2005. doi:10.1042/BJ20050683. PMID 15926889.
- ↑ "Diagnostic value of adenosine deaminase in nontuberculous lymphocytic pleural effusions". Eur. Respir. J. 21 (2): 220–4. 2003. doi:10.1183/09031936.03.00051603. PMID 12608433. http://erj.ersjournals.com/content/21/2/220.full.pdf.
- ↑ Brunicardi, F.; Andersen, Dana; Billiar, Timothy; Dunn, David; Hunter, John; Pollock, Raphael E. (2005). "Chapter 18, question 16". Schwartz's principles of surgery (8th ed.). New York: McGraw-Hill Professional. ISBN 978-0071410908.
Further reading
- "[Adenosine deaminase. A pluridisciplinary enzyme]". Acta Médica Portuguesa 4 (6): 315–23. 1992. PMID 1807098.
- "Cell surface adenosine deaminase: much more than an ectoenzyme". Progress in Neurobiology 52 (4): 283–94. Jul 1997. doi:10.1016/S0301-0082(97)00013-0. PMID 9247966.
- "HIV-1 Envelope gp120 and Viral Particles Block Adenosine Deaminase Binding to Human CD26". Cellular Peptidases in Immune Functions and Diseases. Advances in Experimental Medicine and Biology. 421. 1997. pp. 185–92. doi:10.1007/978-1-4757-9613-1_24. ISBN 978-1-4757-9615-5.
- "Enzymes involved in purine metabolism--a review of histochemical localization and functional implications". Histology and Histopathology 14 (4): 1321–40. Oct 1999. PMID 10506947.
- "Identification of two new missense mutations (R156C and S291L) in two ADA- SCID patients unusual for response to therapy with partial exchange transfusions". Human Mutation 1 (2): 166–8. 1993. doi:10.1002/humu.1380010214. PMID 1284479.
- "Identical 3250-bp deletion between two AluI repeats in the ADA genes of unrelated ADA-SCID patients". Genomics 7 (4): 486–90. Aug 1990. doi:10.1016/0888-7543(90)90190-6. PMID 1696926.
- "Presence of adenosine deaminase on the surface of mononuclear blood cells: immunochemical localization using light and electron microscopy". The Journal of Histochemistry and Cytochemistry 39 (8): 1001–8. Aug 1991. doi:10.1177/39.8.1856451. PMID 1856451.
- "Ecto-enzyme activity of human erythrocyte adenosine deaminase". Molecular and Cellular Biochemistry 86 (2): 135–42. Apr 1989. doi:10.1007/BF00222613. PMID 2770711.
- "Identification of a point mutation resulting in a heat-labile adenosine deaminase (ADA) in two unrelated children with partial ADA deficiency". The Journal of Clinical Investigation 83 (2): 497–501. Feb 1989. doi:10.1172/JCI113909. PMID 2783588.
- "Decreased adenosine deaminase (ADA) and 5'nucleotidase (5NT) activity in peripheral blood T cells in Hodgkin disease". American Journal of Hematology 21 (1): 57–66. Jan 1986. doi:10.1002/ajh.2830210108. PMID 3010705.
- "Complete sequence and structure of the gene for human adenosine deaminase". Biochemistry 25 (25): 8234–44. Dec 1986. doi:10.1021/bi00373a017. PMID 3028473.
- "Mutant human adenosine deaminase alleles and their expression by transfection into fibroblasts". The Journal of Biological Chemistry 263 (31): 16291–6. Nov 1988. doi:10.1016/S0021-9258(18)37591-4. PMID 3182793.
- "Elevated red cell adenosine deaminase activity: a marker of disordered erythropoiesis in Diamond-Blackfan anaemia and other haematologic diseases". British Journal of Haematology 68 (2): 165–8. Feb 1988. doi:10.1111/j.1365-2141.1988.tb06184.x. PMID 3348976.
- "New assignment of the adenosine deaminase gene locus to chromosome 20q13 X 11 by study of a patient with interstitial deletion 20q". Journal of Medical Genetics 24 (2): 93–6. Feb 1987. doi:10.1136/jmg.24.2.93. PMID 3560174.
- "Transient expression of human adenosine deaminase cDNAs: identification of a nonfunctional clone resulting from a single amino acid substitution". Molecular and Cellular Biology 5 (4): 762–7. Apr 1985. doi:10.1128/mcb.5.4.762. PMID 3838797.
- "Adenosine deaminase: characterization and expression of a gene with a remarkable promoter". The EMBO Journal 4 (2): 437–43. Feb 1985. doi:10.1002/j.1460-2075.1985.tb03648.x. PMID 3839456.
- "Identification of a point mutation in the adenosine deaminase gene responsible for immunodeficiency". The Journal of Clinical Investigation 76 (2): 894–7. Aug 1985. doi:10.1172/JCI112050. PMID 3839802.
- "Human adenosine deaminase. cDNA and complete primary amino acid sequence". The Journal of Biological Chemistry 259 (19): 12101–6. Oct 1984. doi:10.1016/S0021-9258(20)71325-6. PMID 6090454.
- "Isolation of cDNA clones for human adenosine deaminase". Gene 25 (2–3): 231–40. Nov 1983. doi:10.1016/0378-1119(83)90227-5. PMID 6198240.
External links
- ADA human gene location in the UCSC Genome Browser.
- ADA human gene details in the UCSC Genome Browser.
- PDBe-KB provides an overview of all the structure information available in the PDB for Human Adenosine deaminase
- PDBe-KB provides an overview of all the structure information available in the PDB for Mouse Adenosine deaminase
 |