Biology:Iodotyrosine deiodinase
| Iodotyrosine deiodinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.21.1.1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
 Generic protein structure example |
Iodotyrosine deiodinase, also known as iodotyrosine dehalogenase 1, is a type of deiodinase enzyme that scavenges iodide by removing it from iodinated tyrosine residues in the thyroid gland.[1] These iodinated tyrosines are produced during thyroid hormone biosynthesis.[2] The iodide that is scavenged by iodotyrosine deiodinase is necessary to again synthesize the thyroid hormones.[3] After synthesis, the thyroid hormones circulate through the body to regulate metabolic rate, protein expression, and body temperature.[4] Iodotyrosine deiodinase is thus necessary to keep levels of both iodide and thyroid hormones in balance.
Dehalogenation in aerobic organisms is usually done through oxidation and hydrolysis;[5] however, iodotyrosine deiodinase uses reductive dehalogenation. Iodotyrosine deiodinase and iodothyronine deiodinase have been determined as the only two known enzymes to catalyze reductive dehalogenation in mammals.[4] Although these two enzymes perform similar functions, they are structurally and mechanistically different. Iodothyronine deiodinase (not the enzyme that is the topic of this article) uses a selenocysteine active site for catalysis, is a member of the thioredoxin superfamily, and removes iodide only when the substrate is in a double-tyrosine form.[6] By contrast, iodotyrosine deiodinase (the topic enzyme) does not require selenocysteine or cysteine for catalysis,[7] is part of the NADH oxidase/flavin reductase superfamily,[8][9] and removes iodide when the substrate is a single amino acid.[10] Research on iodotyrosine deiodinase has historically been variable and slow due to its lack of stability and arduous purification.[11] Only recently has this enzyme been studied more deeply.[4]
Structure

The gene encoding this enzyme has been recently identified.[8][9] The sequence of amino acids of iodotyrosine deiodinase is highly conserved among mammals and contains three domains.[4] Iodotyrosine deiodinase is a membrane protein, with the N-terminus functioning as a membrane anchor.[7][13] It forms a dimer that is domain-swapped.[10] Initially, iodotyrosine deiodinase was thought to contain only one flavin mononucleotide (FMN) in each dimer,[14] but now iodotyrosine deiodinase is believed to have two FMN molecules for each homodimer.[10] The enzyme has a characteristic α-β fold that all proteins from the NADH oxidase/flavin reductase superfamily have as well. Within the dimer interface, there are two equivalent active sites, each made from residues from both subunits. Thus, subunit association must be required for FMN binding and catalysis. Substrate binding causes a conformational change in the enzyme in order to close the active site, protecting the substrate and flavin from the solvent.[10]
Function
Iodotyrosine deiodinase facilitates iodide salvage in the thyroid by catalyzing deiodination of mono- and diiodotyrosine, the halogenated byproducts of thyroid hormone production.[9] Iodide is also an important micronutrient in the biosynthesis of thyroid hormone, creating a cycle of iodide use in the thyroid.[10] Iodide homeostasis within the thyroid gland is essential for producing thyroid hormone at appropriate rates. Thus, iodide levels must be regulated in order to keep thyroid hormones, and ultimately the organism's metabolic rate and overall health, in good status.[15]
Within the thyroid follicular cell, thyroglobulin is hydrolyzed to form thyroid hormones and mono- and diiodotyrosine. The thyroid hormones are released into the bloodstream and the iodinated tyrosines are recycled. However, the breakdown of thyroglobulin produces 6-7 fold more iodinated tyrosines than thyroid hormone.[4] Iodotyrosine deiodinase salvages the iodide from the deiodination of the iodinated tyrosines.[16] Iodotyrosine deiodinase is located on the apical plasma membrane of the thyroid colloid, where mono- and diiodotyrosine are produced from this breakdown of thyroglobulin. Without iodotyrosine deiodinase activity, the iodide would be excreted with the amino acid tyrosine and thyroid hormone biosynthesis would be reduced.[10]
The enzymatic activity of iodotyrosine deiodinase has also been known to exist in the tissues of the liver and kidneys as well;[17] however, the physiological significance of these findings is not yet clear.[4]
Mechanism
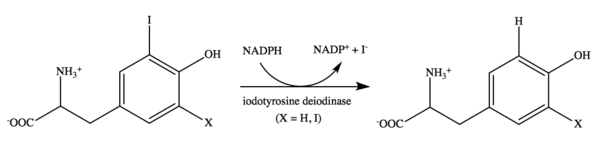
Iodotyrosine deiodinase catalyzes mono- and diiodotyrosine deiodination. The reaction is NADPH-dependent.[13] Flavin mononucleotide (FMN) is a cofactor.[18] Although flavin is commonly utilized in various catalytic reactions,[19] its use in this reductive dehalogenation is unique and not yet fully understood.[10] It is also still unclear if the enzyme mechanism utilizes a two electron transfer reaction or a series of one electron transfers. Although further research must be done to determine details of this mechanism, recent evidence seems to suggest that iodotyrosine deiodinase acts through one electron transfer reactions.[4]
Clinical significance
Mutations in the gene encoding iodotyrosine deiodinase can affect enzyme function and be detrimental to human health. Iodide is an essential micronutrient for health in mammals.[20] Low levels of iodide either through the diet or through iodide metabolism are associated with hypothyroidism, mental retardation, goiter, and developmental defects.[1][4][15] Because iodotyrosine deiodinase is responsible for scavenging iodide, mutations in this enzyme result in iodide deficiency.[21]
The resulting high blood and urine concentrations of iodotyrosine can be used as a measure for diagnosis, as the iodide is not removed from the tyrosine residues effectively.[22] In some countries, newborn babies are tested for congenital hypothyroidism and treated immediately if the disease is detected, safely preventing the development of mental retardation.[23] However, mutations of iodotyrosine deiodinase are often not detected until after developmental damage has already occurred.[15] Furthermore, these mutations may not be specifically detected using standard thyroid function tests.[15] To combat this issue, a sensitive assay has recently been created that measures the amounts of mono- and diiodotyrosine in the urine.[22]
See also
- Thyroid peroxidase
- Iodotyrosine
- Iodothyronine deiodinase
References
- ↑ 1.0 1.1 "Genetics and phenomics of hypothyroidism and goiter due to iodotyrosine deiodinase (DEHAL1) gene mutations". Molecular and Cellular Endocrinology 322 (1–2): 91–8. June 2010. doi:10.1016/j.mce.2010.03.010. PMID 20298747.
- ↑ "Inhibition of thyroidal iodotyrosine deiodination by tyrosine analogues". Endocrinology 83 (2): 336–47. August 1968. doi:10.1210/endo-83-2-336. PMID 5668272.
- ↑ "The metabolism of iodotyrosines. III. Di-iodotyrosine deshalogenating activity of human thyroid tissue". The Journal of Clinical Endocrinology and Metabolism 16 (8): 1096–101. August 1956. doi:10.1210/jcem-16-8-1096. PMID 13345866.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 "Efficient use and recycling of the micronutrient iodide in mammals". Biochimie 92 (9): 1227–35. September 2010. doi:10.1016/j.biochi.2010.02.013. PMID 20167242.
- ↑ Häggblom, M.M.; Bossert, I.D. (2003). Dehalogenation: Microbial Processes and Environmental Applications. Boston: Kluwer Academic Publishers. pp. 520. ISBN 978-1-4757-7807-6.
- ↑ "The iodothyronine selenodeiodinases are thioredoxin-fold family proteins containing a glycoside hydrolase clan GH-A-like structure". The Journal of Biological Chemistry 278 (38): 36887–96. September 2003. doi:10.1074/jbc.M305725200. PMID 12847093.
- ↑ 7.0 7.1 "Flavoprotein iodotyrosine deiodinase functions without cysteine residues". ChemBioChem 9 (4): 504–6. March 2008. doi:10.1002/cbic.200700562. PMID 18228228.
- ↑ 8.0 8.1 "Iodotyrosine dehalogenase 1 (DEHAL1) is a transmembrane protein involved in the recycling of iodide close to the thyroglobulin iodination site". FASEB Journal 18 (13): 1574–6. October 2004. doi:10.1096/fj.04-2023fje. PMID 15289438.
- ↑ 9.0 9.1 9.2 "Iodotyrosine deiodinase is the first mammalian member of the NADH oxidase/flavin reductase superfamily". The Journal of Biological Chemistry 281 (5): 2812–9. February 2006. doi:10.1074/jbc.M510365200. PMID 16316988.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 10.6 "Crystal structure of iodotyrosine deiodinase, a novel flavoprotein responsible for iodide salvage in thyroid glands". The Journal of Biological Chemistry 284 (29): 19659–67. July 2009. doi:10.1074/jbc.M109.013458. PMID 19436071.
- ↑ "Iodotyrosine deiodinase from bovine thyroid". Posttranslational Modifications Part B. Methods in Enzymology. 107. 1984-01-01. pp. 488–500. doi:10.1016/0076-6879(84)07033-6. ISBN 9780121820077.
- ↑ "RCSB PDB - Search Results". RCSB Protein Data Banck. http://www.rcsb.org/pdb/results/results.do?outformat=&qrid=B331BDE0&tabtoshow=Current.
- ↑ 13.0 13.1 "Entrez Gene: IYD Iodotyrosine deiodinase". Entrez Gene. United States National Library of Medicine. https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=389434.
- ↑ "Purification and characterization of a flavoprotein from bovine thyroid with iodotyrosine deiodinase activity". The Journal of Biological Chemistry 254 (24): 12318–25. December 1979. doi:10.1016/S0021-9258(19)86318-4. PMID 500717.
- ↑ 15.0 15.1 15.2 15.3 "Mutations in the iodotyrosine deiodinase gene and hypothyroidism". The New England Journal of Medicine 358 (17): 1811–8. April 2008. doi:10.1056/NEJMoa0706819. PMID 18434651.
- ↑ "An outline of inherited disorders of the thyroid hormone generating system". Thyroid 13 (8): 771–801. August 2003. doi:10.1089/105072503768499671. PMID 14558921.
- ↑ "The syndrome of congenital hypothyroidism with defective dehalogenation of iodotyrosines. Further observations and a discussion of the pathophysiology". The Journal of Clinical Endocrinology and Metabolism 20 (7): 983–1003. July 1960. doi:10.1210/jcem-20-7-983. PMID 13810029.
- ↑ "Characterization of a flavoprotein iodotyrosine deiodinase from bovine thyroid. Flavin nucleotide binding and oxidation-reduction properties". The Journal of Biological Chemistry 254 (24): 12326–30. December 1979. doi:10.1016/S0021-9258(19)86319-6. PMID 500718.
- ↑ "The diverse roles of flavin coenzymes--nature's most versatile thespians". The Journal of Organic Chemistry 72 (17): 6329–42. August 2007. doi:10.1021/jo0703092. PMID 17580897.
- ↑ Eastman, Creswell J.; Zimmermann, Michael (2000-01-01). De Groot, Leslie J.. ed. The Iodine Deficiency Disorders. South Dartmouth (MA): MDText.com, Inc.. https://www.ncbi.nlm.nih.gov/books/NBK285556/.
- ↑ "Inherited disorders of thyroid metabolism". Endocrine Reviews 4 (3): 213–39. 1983. doi:10.1210/edrv-4-3-213. PMID 6354701.
- ↑ 22.0 22.1 "Molecular characterization of iodotyrosine dehalogenase deficiency in patients with hypothyroidism". The Journal of Clinical Endocrinology and Metabolism 93 (12): 4894–901. December 2008. doi:10.1210/jc.2008-0865. PMID 18765512. https://zenodo.org/record/894080.
- ↑ "Effects of neonatal screening for hypothyroidism: prevention of mental retardation by treatment before clinical manifestations.". Lancet 2 (8255): 1095–8. November 1981. doi:10.1016/s0140-6736(81)91287-3. PMID 6118534.
Further reading
- "Identification of novel genes involved in congenital hypothyroidism using serial analysis of gene expression". Hormone Research 60 Suppl 3 (3): 96–102. 2003. doi:10.1159/000074509. PMID 14671405.
- "Molecular characterization of iodotyrosine dehalogenase deficiency in patients with hypothyroidism". The Journal of Clinical Endocrinology and Metabolism 93 (12): 4894–901. December 2008. doi:10.1210/jc.2008-0865. PMID 18765512. https://zenodo.org/record/894080.
- Gibson, Greg, ed (November 2008). "Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum". PLOS Genetics 4 (11): e1000282. doi:10.1371/journal.pgen.1000282. PMID 19043545.
- "Crystal structure of iodotyrosine deiodinase, a novel flavoprotein responsible for iodide salvage in thyroid glands". The Journal of Biological Chemistry 284 (29): 19659–67. July 2009. doi:10.1074/jbc.M109.013458. PMID 19436071.
- "Characterisation of DEHAL1 expression in thyroid pathologies". European Journal of Endocrinology 156 (3): 295–301. March 2007. doi:10.1530/EJE-06-0596. PMID 17322488.
- "Cloning and characterization of a novel isoform of iodotyrosine dehalogenase 1 (DEHAL1) DEHAL1C from human thyroid: comparisons with DEHAL1 and DEHAL1B". Thyroid 16 (8): 715–24. August 2006. doi:10.1089/thy.2006.16.715. PMID 16910871.
- "Genome-wide association study of panic disorder in the Japanese population". Journal of Human Genetics 54 (2): 122–6. February 2009. doi:10.1038/jhg.2008.17. PMID 19165232.
External links
- Iodotyrosine+Deiodinase at the US National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: Q6PHW0 (Human Iodotyrosine deiodinase 1) at the PDBe-KB.
- Overview of all the structural information available in the PDB for UniProt: Q9DCX8 (Mouse Iodotyrosine deiodinase 1) at the PDBe-KB.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
 |

