Biology:TEV protease
| nuclear-inclusion-a endopeptidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 | |||||||||
| Identifiers | |||||||||
| EC number | 3.4.22.44 | ||||||||
| CAS number | 139946-51-3 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
TEV protease (EC 3.4.22.44, Tobacco Etch Virus nuclear-inclusion-a endopeptidase) is a highly sequence-specific cysteine protease from Tobacco Etch Virus (TEV).[1] It is a member of the PA clan of chymotrypsin-like proteases.[2] Due to its high sequence specificity, TEV protease is frequently used for the controlled cleavage of fusion proteins in vitro and in vivo.[3]
Origin
The tobacco etch virus encodes its entire genome as a single massive polyprotein (350 kDa). This is cleaved into functional units by the three proteases: P1 protease (1 cleavage site), helper-component protease (1 cleavage site) and TEV protease (7 cleavage sites).[1] The native TEV protease also contains an internal self-cleavage site. This site is slowly cleaved to inactivate the enzyme (the physiological reason for this is unknown).
Structure and function
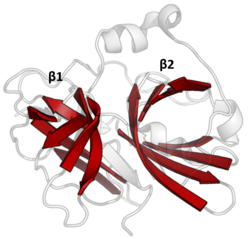
The structure of TEV protease has been solved by X-ray crystallography.[4] It is composed of two β-barrels and a flexible C-terminal tail and displays structural homology to the chymotrypsin superfamily of proteases (PA clan, C4 family by MEROPS classification).[2] Although homologous to cellular serine proteases (such as trypsin, elastase, thrombin etc.), TEV protease uses a cysteine as its catalytic nucleophile[5] (as do many other viral proteases).
Covalent catalysis is performed with an Asp-His-Cys triad, split between the two barrels (Asp on β1 and His and Cys on β2).[6] The substrate is held as a β-sheet, forming an antiparallel interaction with the cleft between the barrels and a parallel interaction with the C-terminal tail.[7] The enzyme therefore forms a binding tunnel around the substrate and side chain interactions control specificity.[4]
Specificity
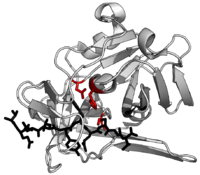
The preferred, native cleavage sequence was first identified by examining the cut sites in the native polyprotein substrate for recurring sequence. The consensus for these native cut sites is ENLYFQ\S where ‘\’ denotes the cleaved peptide bond.[8] Residues of the substrate are labelled P6 to P1 before the cut site and P1’ after the cut site. Early works also measured cleavage of an array of similar substrates to characterise how specific the protease was for the native sequence.[9][10]
Studies have subsequently used sequencing of cleaved substrates from a pool of randomised sequences to determine preference patterns.[11][12] Although ENLYFQ\S is the optimal sequence, the protease is active to a greater or lesser extent on a range of substrates (i.e. shows some substrate promiscuity). The highest cleavage is of sequences closest to the consensus EXLYΦQ\φ where X is any residue, Φ is any large or medium hydrophobe and φ is any small hydrophobic or polar residue. Although this sequence is the optimal, sequences with disfavoured residues at some positions can still be cleaved if the rest of the sequence is optimal.[10][12]
Specificity is endowed by the large contact area between enzyme and substrate. Proteases such as trypsin have specificity for one residue before and after the cleaved bond due to a shallow binding cleft with only one or two pockets that bind the substrate side chains. Conversely, viral proteases such as TEV protease have a long C-terminal tail which completely covers the substrate to create a binding tunnel. This tunnel contains a set of tight binding pockets such that each side chain of the substrate peptide (P6 to P1’) is bound in a complementary site (S6 to S1’).[4]
In particular, peptide side chain P6-Glu contacts a network of three hydrogen bonds; P5-Asn points into the solvent, making no specific interactions (hence the absence of substrate consensus at this position); P4-Leu is buried in a hydrophobic pocket; P3-Tyr is held in a hydrophobic pocket with a short hydrogen bond at the end; P2-Phe is also surrounded by hydrophobes including the face of the triad histidine; P1-Gln forms four hydrogen bonds; and P1’-Ser is only partly enclosed in a shallow hydrophobic groove.[4]
Application as a biochemical tool
One of the main uses of this protein is for removing affinity tags from purified recombinant fusion proteins. The reason for the use of TEV protease as a biochemical tool is its high sequence specificity. This specificity allows for the controlled cleavage of proteins when the preference sequence is inserted into flexible loops. It also makes TEV protease relatively non-toxic in vivo as the recognized sequence scarcely occurs in proteins.[13]
Although rational design has had limited success in changing protease specificity, directed evolution has been used to change the preferred residue either before[14] or after[15][16] the cleavage site.
However, TEV protease does have limitations as a biochemical tool. It is prone to deactivation by self-cleavage (autolysis), though this can be abolished through a single S219V mutation in the internal cleavage site.[17] The protease expressed alone is also poorly soluble, however several attempts have been made to improve its solubility through directed evolution and computational design. It has also been shown that expression can be improved by fusion to maltose binding protein (MBP) which acts a solubility-enhancing partner.[3]
TEV protease has been reported to show a 10-fold loss of activity at 4 °C.[18] TEV protease shows loss of activity at temperatures above 34 °C.[19] The original TEV protease required the presence of reducing agent for high activity, which could interfere with the function of proteins containing disulfide bonds. After incorporation of various mutations, later "superTEV protease" versions are highly active in the presence or absence of reducing agent.[20][21][22]
The molecular weight of this enzyme varies between 25 and 27 kDa depending on the specific construct used.
References
- ↑ 1.0 1.1 UniProt: TEV polyprotein: "P04517". https://www.uniprot.org/uniprot/P04517.
- ↑ 2.0 2.1 "MEROPS: the database of proteolytic enzymes, their substrates and inhibitors". Nucleic Acids Res. 40 (Database issue): D343–50. January 2012. doi:10.1093/nar/gkr987. PMID 22086950.
- ↑ 3.0 3.1 "Controlled intracellular processing of fusion proteins by TEV protease". Protein Expr. Purif. 19 (2): 312–8. July 2000. doi:10.1006/prep.2000.1251. PMID 10873547.
- ↑ 4.0 4.1 4.2 4.3 "Structural basis for the substrate specificity of tobacco etch virus protease". J. Biol. Chem. 277 (52): 50564–72. December 2002. doi:10.1074/jbc.M207224200. PMID 12377789.
- ↑ "Viral cysteine proteases are homologous to the trypsin-like family of serine proteases: structural and functional implications". Proc. Natl. Acad. Sci. U.S.A. 85 (21): 7872–6. November 1988. doi:10.1073/pnas.85.21.7872. PMID 3186696. Bibcode: 1988PNAS...85.7872B.
- ↑ "Characterization of the catalytic residues of the tobacco etch virus 49-kDa proteinase". Virology 172 (1): 302–10. September 1989. doi:10.1016/0042-6822(89)90132-3. PMID 2475971.
- ↑ "Proteases universally recognize beta strands in their active sites". Chem. Rev. 105 (3): 973–99. March 2005. doi:10.1021/cr040669e. PMID 15755082.
- ↑ "A viral cleavage site cassette: identification of amino acid sequences required for tobacco etch virus polyprotein processing". Proc. Natl. Acad. Sci. U.S.A. 85 (10): 3391–5. May 1988. doi:10.1073/pnas.85.10.3391. PMID 3285343. Bibcode: 1988PNAS...85.3391C.
- ↑ "Molecular genetic analysis of a plant virus polyprotein cleavage site: a model". Virology 171 (2): 356–64. August 1989. doi:10.1016/0042-6822(89)90603-X. PMID 2669323.
- ↑ 10.0 10.1 Kapust, Rachel B.; Tözsér, József; Copeland, Terry D.; Waugh, David S. (2002-06-28). "The P1' specificity of tobacco etch virus protease". Biochemical and Biophysical Research Communications 294 (5): 949–955. doi:10.1016/S0006-291X(02)00574-0. ISSN 0006-291X. PMID 12074568.
- ↑ "Evolutionary optimization of peptide substrates for proteases that exhibit rapid hydrolysis kinetics". Biotechnol. Bioeng. 106 (3): 339–46. June 2010. doi:10.1002/bit.22693. PMID 20148412.
- ↑ 12.0 12.1 "Substrate profiling of tobacco etch virus protease using a novel fluorescence-assisted whole-cell assay". PLOS ONE 6 (1): e16136. 2011. doi:10.1371/journal.pone.0016136. PMID 21267463. Bibcode: 2011PLoSO...616136K.
- ↑ "Release of proteins and peptides from fusion proteins using a recombinant plant virus proteinase". Anal. Biochem. 216 (2): 413–7. February 1994. doi:10.1006/abio.1994.1060. PMID 8179197.
- ↑ "Engineering of TEV protease variants by yeast ER sequestration screening (YESS) of combinatorial libraries". Proc. Natl. Acad. Sci. U.S.A. 110 (18): 7229–34. April 2013. doi:10.1073/pnas.1215994110. PMID 23589865. Bibcode: 2013PNAS..110.7229Y.
- ↑ "A tobacco etch virus protease with increased substrate tolerance at the P1' position". PLOS ONE 8 (6): e67915. 2013. doi:10.1371/journal.pone.0067915. PMID 23826349. Bibcode: 2013PLoSO...867915R.
- ↑ "Intracellular detection and evolution of site-specific proteases using a genetic selection system". Appl. Biochem. Biotechnol. 166 (5): 1340–54. March 2012. doi:10.1007/s12010-011-9522-6. PMID 22270548.
- ↑ "Tobacco etch virus protease: mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency". Protein Eng. 14 (12): 993–1000. December 2001. doi:10.1093/protein/14.12.993. PMID 11809930.
- ↑ "Differential temperature dependence of tobacco etch virus and rhinovirus 3C proteases". Analytical Biochemistry 436 (2): 142–144. 15 May 2013. doi:10.1016/j.ab.2013.01.031. PMID 23395976.
- ↑ "Efficient site-specific processing of fusion proteins by tobacco vein mottling virus protease in vivo and in vitro". Protein Expr. Purif. 38 (1): 108–115. November 2004. doi:10.1016/j.pep.2004.08.016. PMID 15477088.
- ↑ "Enhancing the stability and solubility of TEV protease using in silico design". Protein Sci. 16 (11): 2360–7. 2007. doi:10.1110/ps.072822507. PMID 17905838.
- ↑ "Screening, large-scale production and structure-based classification of cystine-dense peptides.". Nat Struct Mol Biol 25 (3): 270–278. 2018. doi:10.1038/s41594-018-0033-9. PMID 29483648.
- ↑ "Approaching infinite affinity through engineering of peptide-protein interaction". Proceedings of the National Academy of Sciences of the United States of America 116 (52): 26523–26533. December 2019. doi:10.1073/pnas.1909653116. PMID 31822621. Bibcode: 2019PNAS..11626523K.
External links
 |