Biology:Aziridines
thumb|right|220 px|[[Mitomycin C, an aziridine, is used as a chemotherapeutic agent by virtue of its antitumour activity.[1]]]
In organic chemistry, aziridines are organic compounds containing the aziridine functional group (chemical structure (R–)
4C
2N–R), a three-membered heterocycle with one amine (>NR) and two methylene bridges (>CR
2).[2][3][4] The parent compound is aziridine (or ethylene imine), with molecular formula C
2H
4NH. Several drugs feature aziridine rings, including mitomycin C, porfiromycin, and azinomycin B (carzinophilin).[5]
Structure
The bond angles in aziridine are approximately 60°, considerably less than the normal hydrocarbon bond angle of 109.5°, which results in angle strain as in the comparable cyclopropane and ethylene oxide molecules. A banana bond model explains bonding in such compounds. Aziridine is less basic than acyclic aliphatic amines, with a pKa of 7.9 for the conjugate acid, due to increased s character of the nitrogen free electron pair. Angle strain in aziridine also increases the barrier to nitrogen inversion. This barrier height permits the isolation of separate invertomers, for example the cis and trans invertomers of N-chloro-2-methylaziridine.
Synthesis
Several routes have been developed for the syntheses of aziridines (aziridination).
Cyclization of haloamines and amino alcohols
An amine functional group displaces the adjacent halide in an intramolecular nucleophilic substitution reaction to generate an aziridine. The parent aziridine is produced industrially from aminoethanol via two related routes. The Nippon Shokubai process requires an oxide catalyst and high temperatures to effect the dehydration. In the Wenker synthesis, the aminoethanol is converted to the sulfate ester, which undergoes base-induced sulfate elimination.[6]
Nitrene addition
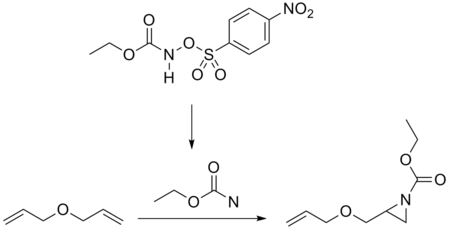
Nitrene addition to alkenes is a well-established method for the synthesis of aziridines. Photolysis or thermolysis of organic azides are good ways to generate nitrenes. Nitrenes can also be prepared in situ from iodosobenzene diacetate and sulfonamides, or the ethoxycarbonylnitrene from the N-sulfonyloxy precursor.[7]
From triazolines, epoxides, and oximes
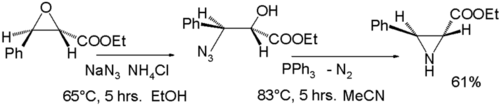
Thermolysis or photolysis of triazolines expels nitrogen, producing an aziridine. One method involves the ring-opening reaction of an epoxide with sodium azide, followed by reduction of the azide with triphenylphosphine accompanied by expulsion of nitrogen gas:[8]
Another method involves the ring-opening reaction of an epoxide with amines, followed by ring closing with the Mitsunobu reaction.[9]
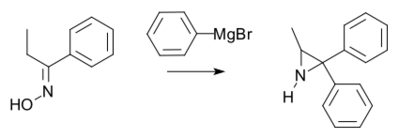
The Hoch-Campbell ethylenimine synthesis involves the reaction of certain oximes with Grignard reagents, which affords aziridines.[10]
From alkenes using DPH
Aziridines are obtained by treating a mono-, di-, tri- or tetra-substituted alkene (olefin) with O-(2,4-dinitrophenyl)hydroxylamine (de) in the presence of rhodium catalysts. [math]\ce{ \mathrm{Alkene} + \mathrm{DPH} ->[{}\atop\ce{Rh2(CO2R)4}] \mathrm{Aziridine} }[/math]
For instance, Ph-Aziridine-Me can be synthesized by this method and then converted by ring opening reaction to (D)- and (L)-amphetamine (the two active ingredients in Adderall).[11]
From α-chloroimines
The De Kimpe aziridine synthesis allows for the generation of aziridines by reacting an α-chloroimine with a nucleophile, such as hydride, cyanide, or a Grignard reagent.[12][13]
From 2-azido alcohols
2-azido alcohols can be converted into aziridines with the use of trialkyl phosphines such as trimethylphosphine or tributylphosphine.[14][15]
Reactions
Nucleophilic ring opening
Aziridines are reactive substrates in ring-opening reactions with many nucleophiles due to their ring strain. Alcoholysis and aminolysis are basically the reverse reactions of the cyclizations. Carbon nucleophiles such as organolithium reagents and organocuprates are also effective.[16][17]
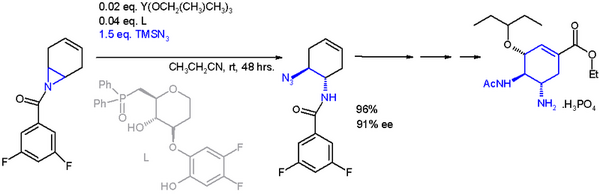
One application of a ring-opening reaction in asymmetric synthesis is that of trimethylsilylazide TMSN3 with an asymmetric ligand[18] in scheme 2[19] in an organic synthesis of oseltamivir:
1,3-dipole formation
Certain N-substituted azirines with electron withdrawing groups on both carbons form azomethine ylides in an electrocyclic thermal or photochemical ring-opening reaction.[20][21] These ylides can be trapped with a suitable dipolarophile in a 1,3-dipolar cycloaddition.[22]
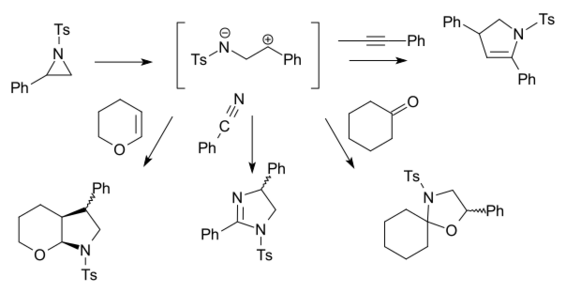
When the N-substituent is an electron-withdrawing group such as a tosyl group, the carbon-nitrogen bond breaks, forming another zwitterion TsN−–CH2–CH+2–R[23]
This reaction type requires a Lewis acid catalyst such as boron trifluoride. In this way 2-phenyl-N-tosylaziridine reacts with alkynes, nitriles, ketones and alkenes. Certain 1,4-dipoles form from azetidines.
Other
Lewis acids, such as B(C6F5)3, can induce decomposition of the ring to a carbocation and linear azanide, which then attack unsaturated moieties in tandem.[24] Oxidation to the N-oxide instead induces nitroso compound extrusion, leaving an olefin.[25]
Safety
As electrophiles, aziridines are subject to attack and ring-opening by endogenous nucleophiles such as nitrogenous bases in DNA base pairs, resulting in potential mutagenicity.[26][27][28]
The International Agency for Research on Cancer (IARC) classifies aziridine compounds as possibly carcinogenic to humans (IARC Group 2B).[29] In making the overall evaluation, the IARC Working Group took into consideration that aziridine is a direct-acting alkylating agent, which is mutagenic in a wide range of test systems and forms DNA adducts that are promutagenic. The features that are responsible for their mutagenicity are relevant to their beneficial medicinal properties.[5]
See also
- Binary ethylenimine, a dimeric form of aziridine
References
- ↑ Tomasz, Maria (September 1995). "Mitomycin C: small, fast and deadly (but very selective).". Chemistry and Biology 2 (9): 575–579. doi:10.1016/1074-5521(95)90120-5. PMID 9383461.
- ↑ Gilchrist, T.L. (1987). Heterocyclic chemistry. ISBN 978-0-582-01421-3.
- ↑ Epoxides and Aziridines – A Mini Review Albert Padwa and S. Shaun Murphree Arkivoc (JC-1522R) pp. 6–33 Online article
- ↑ Sweeney, J. B. (2002). "Aziridines: Epoxides' ugly cousins?". Chem. Soc. Rev. 31 (5): 247–258. doi:10.1039/B006015L. PMID 12357722.
- ↑ 5.0 5.1 Ismail, Fyaz M.D.; Levitsky, Dmitri O.; Dembitsky, Valery M. (2009). "Aziridine alkaloids as potential therapeutic agents". European Journal of Medicinal Chemistry 44 (9): 3373–3387. doi:10.1016/j.ejmech.2009.05.013. PMID 19540628.
- ↑ Steuerle, Ulrich; Feuerhake, Robert (2006). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_239.pub2.
- ↑ M. Antonietta Loreto; Lucio Pellacani; Paolo A. Tardella; Elena Toniato (1984). "Addition reactions of ethoxycarbonylnitrene and ethoxycarbonylnitrenium ion to allylic ethers". Tetrahedron Letters 25 (38): 4271–4. doi:10.1016/S0040-4039(01)81414-3.
- ↑ Ryan Hili; Andrei K. Yudin (2006). "Readily Available Unprotected Amino Aldehydes". J. Am. Chem. Soc. 128 (46): 14772–3. doi:10.1021/ja065898s. PMID 17105264.
- ↑ B. Pulipaka; Stephen C. Bergmeier (2008). "Synthesis of Hexahydro-1 H -benzo[ c ]chromen-1-amines via the Intramolecular Ring-Opening Reof Aziridines by π-Nucleophiles". Synthesis 2008 (9): 1420–30. doi:10.1055/s-2008-1072561.
- ↑ "Hoch-Campbell Reaction". Comprehensive Organic Name Reactions and Reagents. 2010. doi:10.1002/9780470638859.conrr320. ISBN 9780470638859.
- ↑ Jat, Jawahar L.; Paudyal, Mahesh P.; Gao, Hongyin; Xu, Qing-Long; Yousufuddin, Muhammed; Devarajan, Deepa; Ess, Daniel H.; Kürti, László et al. (2014-01-03). "Direct Stereospecific Synthesis of Unprotected N-H and N-Me Aziridines from Olefins" (in en). Science 343 (6166): 61–65. doi:10.1126/science.1245727. ISSN 0036-8075. PMID 24385626. Bibcode: 2014Sci...343...61J.
- ↑ De Kimpe, Norbert; Moens, Luc (6 February 1990). "Synthesis of 1,2,3-trisubstituted and 1,2,2,3-tetrasubstituted aziridines from α-chloroketimines". Tetrahedron 46 (8): 2965–2974. doi:10.1016/S0040-4020(01)88388-5.
- ↑ "Asymmetric Synthesis of Aziridines by Reduction of N-tert-Butanesulfinyl α-Chloro Imines". The Journal of Organic Chemistry 72 (9): 3211–3217. 31 March 2007. doi:10.1021/jo0624795. PMID 17397222.
- ↑ Ittah, Ytzhak; Sasson, Yoel; Shahak, Israel; Tsaroom, Shalom; Blum, Jochanan (1 October 1978). "A new aziridine synthesis from 2-azido alcohols and tertiary phosphines. Preparation of phenanthrene 9,10-imine". The Journal of Organic Chemistry 43 (22): 4271–4273. doi:10.1021/jo00416a003.
- ↑ Breuning, Alexander; Vicik, Radim; Schirmeister, Tanja (31 October 2003). "An improved synthesis of aziridine-2,3-dicarboxylates via azido alcohols—epimerization studies". Tetrahedron: Asymmetry 14 (21): 3301–3312. doi:10.1016/j.tetasy.2003.09.015.
- ↑ Hu, X.Eric (2004). "Nucleophilic ring opening of aziridines". Tetrahedron 60 (12): 2701–2743. doi:10.1016/j.tet.2004.01.042.
- ↑ McCoull, William; Davis, Franklin A. (2000). "Recent Synthetic Applications of Chiral Aziridines". Synthesis 2000 (10): 1347–1365. doi:10.1055/s-2000-7097.
- ↑ Yuhei Fukuta; Tsuyoshi Mita; Nobuhisa Fukuda; Motomu Kanai; Masakatsu Shibasaki (2006). "De Novo Synthesis of Tamiflu via a Catalytic Asymmetric Ring-Opening of meso-Aziridines with TMSN3". J. Am. Chem. Soc. 128 (19): 6312–3. doi:10.1021/ja061696k. PMID 16683784.
- ↑ The catalyst is based on yttrium with three isopropyloxy substituents and the ligand a phosphine oxide (Ph = phenyl), with 91% enantiomeric excess (ee)
- ↑ Harold W. Heine; Richard Peavy (1965). "Aziridines XI. Reaction of 1,2,3-triphenylaziridine with diethylacetylene dicarboxylate and maleic anhydride". Tetrahedron Letters 6 (35): 3123–6. doi:10.1016/S0040-4039(01)89232-7.
- ↑ Albert Padwa; Lewis Hamilton (1965). "Reactions of aziridines with dimethylacetylene dicarboxylate". Tetrahedron Letters 6 (48): 4363–7. doi:10.1016/S0040-4039(00)71101-4.
- ↑ Philippe Dauban; Guillaume Malik (2009). "A Masked 1,3-Dipole Revealed from Aziridines". Angew. Chem. Int. Ed. 48 (48): 9026–9. doi:10.1002/anie.200904941. PMID 19882612.
- ↑ Ioana Ungureanua; Cristian Bologab; Saïd Chayera; André Mann (16 July 1999). "Phenylaziridine as a 1,3-dipole. Application to the synthesis of functionalized pyrrolidines". Tetrahedron Letters 40 (29): 5315–8. doi:10.1016/S0040-4039(99)01002-3.
- ↑ Aravinda B. Pulipaka; Stephen C. Bergmeier (2008). "A Synthesis of 6-Azabicyclo[3.2.1]octanes. The Role of N-Substitution". J. Org. Chem. 73 (4): 1462–7. doi:10.1021/jo702444c. PMID 18211092.
- ↑ Hata, Yoshiteru; Watanabe, Masamichi (January 1994). "Metabolism of Aziridines and the Mechanism of their Cytotoxicity" (in en). Drug Metabolism Reviews 26 (3): 575–604. doi:10.3109/03602539408998318. ISSN 0360-2532. http://www.tandfonline.com/doi/full/10.3109/03602539408998318.
- ↑ "Occupational respiratory and skin sensitization caused by polyfunctional aziridine hardener". Clin Exp Allergy 25 (5): 432–9. May 1995. doi:10.1111/j.1365-2222.1995.tb01074.x. PMID 7553246.
- ↑ "Skin and respiratory allergic disease caused by polyfunctional aziridine". Med Lav 94 (3): 285–95. 2003. PMID 12918320.
- ↑ Mapp CE (2001). "Agents, old and new, causing occupational asthma". Occup. Environ. Med. 58 (5): 354–60. doi:10.1136/oem.58.5.354. PMID 11303086.
- ↑ Some Aziridines, N-, S- and O-Mustards and Selenium. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 9. 1975. ISBN 978-92-832-1209-6. http://monographs.iarc.fr/ENG/Monographs/vol9/volume9.pdf. Retrieved 2019-11-24.
 |