Chemistry:Adderall
Adderall is the brand name of a fixed-dose combination medication used for the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy.[1] It is also used as an athletic performance enhancer, cognitive enhancer, appetite suppressant, and recreationally as a euphoriant. Such uses are illegal in many countries. It is a central nervous system (CNS) stimulant of the phenethylamine class.[2][3] It contains the amphetamines dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate.[1] It is taken by mouth.[1]
In therapeutic doses, Adderall causes emotional and cognitive effects such as euphoria, change in sex drive, increased wakefulness, and improved cognitive control. At these doses, it induces physical effects such as a faster reaction time, fatigue resistance, and increased muscle strength. In contrast, much larger doses of Adderall can impair cognitive control, cause rapid muscle breakdown, provoke panic attacks, or induce psychosis (e.g., paranoia, delusions, hallucinations). The side effects vary widely among individuals but most commonly include insomnia, dry mouth, loss of appetite and weight loss. The routine use of Adderall at higher-than-prescribed doses poses a significant risk of addiction or dependence due to the pronounced reinforcing effects that are present at high doses. Recreational doses of Adderall are generally much larger than prescribed therapeutic doses and also carry a far greater risk of serious adverse effects.[sources 1]
The two amphetamine enantiomers that compose Adderall, such as Adderall tablets/capsules (levoamphetamine and dextroamphetamine), alleviate the symptoms of ADHD and narcolepsy by increasing the activity of the neurotransmitters norepinephrine and dopamine in the brain, which results in part from their interactions with human trace amine-associated receptor 1 (hTAAR1) and vesicular monoamine transporter 2 (VMAT2) in neurons. Dextroamphetamine is a more potent CNS stimulant than levoamphetamine, but levoamphetamine has slightly stronger cardiovascular and peripheral effects and a longer elimination half-life than dextroamphetamine. The active ingredient in Adderall, amphetamine, shares many chemical and pharmacological properties with the human trace amines, particularly phenethylamine and N-methylphenethylamine, the latter of which is a positional isomer of amphetamine.[sources 2] In 2023, Adderall was the fifteenth most commonly prescribed medication in the United States, with more than 32 million prescriptions.[22][23]
Uses
Medical
Adderall is indicated for the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy.[1][2]
Attention Deficit Hyperactivity Disorder
{{#lsth:Amphetamine|ADHD}}
Narcolepsy
{{#lsth:Amphetamine|Narcolepsy}}
Available forms
Adderall is available as immediate-release (IR) tablets and extended-release (XR) capsules.[1][24] Mydayis is available as an extended-release formulation.[25] Adderall XR is approved to treat attention deficit hyperactivity disorder for up to 12 hours in individuals 6 years and older and uses a double-bead formulation. The capsule can be swallowed like a tablet, or it can be opened and the beads sprinkled over applesauce for comparable absorption.[24] Upon ingestion, half of the beads provide immediate administration of medication, while the other half are enveloped in a coating that must dissolve, delaying absorption of its contents. It is designed to provide a therapeutic effect and plasma concentrations identical to taking two doses of Adderall IR four hours apart.[24] Mydayis uses a longer-lasting triple-bead formulation and is approved to treat attention deficit hyperactivity disorder for up to 16 hours in individuals aged 13 years of age and older.[25] In the United States, the immediate and extended-release formulations of Adderall are available as generic medications.[26][27] Generic formulations of Mydayis became available in the US in October 2023.[28]
Enhancing performance
{{#section-h:Amphetamine|Enhancing performance}}
Adderall is banned by the National Football League (NFL), Major League Baseball (MLB), the National Basketball Association (NBA), the National Collegiate Athletic Association (NCAA), and the National Hockey League (NHL).[29] In leagues such as the National Football League, there is a very rigorous process required to obtain an exemption to this rule even when the athlete has been medically prescribed the drug by their physician.[29]
Recreational
Adderall has a high potential for misuse as a recreational drug.[30][31][32] Adderall tablets can either be swallowed, crushed and snorted, or dissolved in water and injected.[33] Injection into the bloodstream can be dangerous because insoluble fillers within the tablets can block small blood vessels.[33]
Many postsecondary students have reported using Adderall for study purposes in different parts of the developed world.[32] Among these students, some of the risk factors for misusing ADHD stimulants recreationally include: Inadequate accommodation of disability, basing one's self-worth on external validation, low self-efficacy, earning poor grades, and having an untreated mental health disorder.[32]
Contraindications
Adverse effects
The adverse side effects of Adderall are many and varied, but the amount of substance consumed is the primary factor in determining the likelihood and severity of side effects.[7][18] Adderall is currently approved for long-term therapeutic use by the USFDA.[7] Recreational use of Adderall generally involves far larger doses and is therefore significantly more dangerous, involving a much greater risk of serious adverse drug effects than dosages used for therapeutic purposes.[18] {{#section-h:Amphetamine|Adverse effects}}
Overdose
Interactions
- Monoamine oxidase inhibitors (MAOIs) taken with amphetamine may result in a hypertensive crisis if taken within two weeks after last use of an MAOI type drug.[24]
- Inhibitors of enzymes that directly metabolize amphetamine (particularly CYP2D6 and FMO3) will prolong the elimination of amphetamine and increase drug effects.[24][34][25]
- Serotonergic drugs (such as most antidepressants) co-administered with amphetamine increases the risk of serotonin syndrome.[25]
- Stimulants and antidepressants (sedatives and depressants) may increase (decrease) the drug effects of amphetamine, and vice versa.[24]
- Gastrointestinal and urinary pH affect the absorption and elimination of amphetamine, respectively. Gastrointestinal alkalinizing agents increase the absorption of amphetamine. Urinary alkalinizing agents increase the concentration of non-ionized species, decreasing urinary excretion.[24]
- Proton-pump inhibitors (PPIs) modify the absorption of Adderall XR and Mydayis.[24][25]
- Zinc supplementation may reduce the minimum effective dose of amphetamine when it is used for the treatment of ADHD.[note 1][38]
- Norepinephrine reuptake inhibitors (NRIs) like atomoxetine prevent norepinephrine release induced by amphetamines and have been found to reduce the stimulant, euphoriant, and sympathomimetic effects of dextroamphetamine in humans.[39][40][41]
Pharmacology
Mechanism of action
| Compound | NE | DA | 5-HT | Template:Reference column heading | ||
|---|---|---|---|---|---|---|
| Phenethylamine | 10.9 | 39.5 | >10000 | [42][43][44] | ||
| Dextroamphetamine | 6.6–7.2 | 5.8–24.8 | 698–1765 | [45][46] | ||
| Levoamphetamine | 9.5 | 27.7 | ND | [43][44] | ||
| Dextromethamphetamine | 12.3–13.8 | 8.5–24.5 | 736–1291.7 | [45][47] | ||
| Levomethamphetamine | 28.5 | 416 | 4640 | [45] | ||
| Notes: The smaller the value, the more strongly the drug releases the neurotransmitter. See also Monoamine releasing agent § Activity profiles for a larger table with more compounds. Refs: [48][49] | ||||||
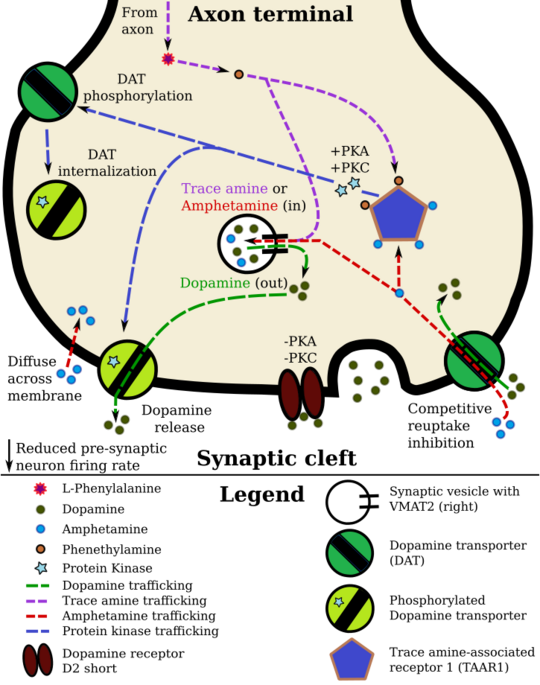
Pharmacodynamics of amphetamine in a dopamine neuron
|
Amphetamine, the active ingredient of Adderall, works primarily by increasing the activity of the neurotransmitters dopamine and norepinephrine in the brain.[14][55] It also triggers the release of several other hormones (e.g., epinephrine) and neurotransmitters (e.g., serotonin and histamine) as well as the synthesis of certain neuropeptides (e.g., cocaine and amphetamine regulated transcript (CART) peptides).[16][56] Both active ingredients of Adderall, dextroamphetamine and levoamphetamine, bind to the same biological targets,[18][19] but their binding affinities (that is, potency) differ somewhat.[18][19] Dextroamphetamine and levoamphetamine are both potent full agonists (activating compounds) of trace amine-associated receptor 1 (TAAR1) and interact with vesicular monoamine transporter 2 (VMAT2), with dextroamphetamine being the more potent agonist of TAAR1.[19] Consequently, dextroamphetamine produces more CNS stimulation than levoamphetamine;[19][57] however, levoamphetamine has slightly greater cardiovascular and peripheral effects.[18] It has been reported that certain children have a better clinical response to levoamphetamine.[20][21]
In the absence of amphetamine, VMAT2 will normally move monoamines (e.g., dopamine, histamine, serotonin, norepinephrine, etc.) from the intracellular fluid of a monoamine neuron into its synaptic vesicles, which store neurotransmitters for later release (via exocytosis) into the synaptic cleft.[16] When amphetamine enters a neuron and interacts with VMAT2, the transporter reverses its direction of transport, thereby releasing stored monoamines inside synaptic vesicles back into the neuron's intracellular fluid.[16] Meanwhile, when amphetamine activates TAAR1, the receptor causes the neuron's cell membrane-bound monoamine transporters (i.e., the dopamine transporter, norepinephrine transporter, or serotonin transporter) to either stop transporting monoamines altogether (via transporter internalization) or transport monoamines out of the neuron;[15] in other words, the reversed membrane transporter will push dopamine, norepinephrine, and serotonin out of the neuron's intracellular fluid and into the synaptic cleft.[15] In summary, by interacting with both VMAT2 and TAAR1, amphetamine releases neurotransmitters from synaptic vesicles (the effect from VMAT2) into the intracellular fluid where they subsequently exit the neuron through the membrane-bound, reversed monoamine transporters (the effect from TAAR1).[15][16]
Pharmacokinetics
{{#section-h:Amphetamine|Pharmacokinetics}}
Pharmacomicrobiomics
Related endogenous compounds
{{#section-h:Amphetamine|Related endogenous compounds}}
History
The pharmaceutical company Rexar reformulated their weight loss drug Obetrol following its mandatory withdrawal from the market in 1973, under the Kefauver Harris Amendment to the Federal Food, Drug, and Cosmetic Act due to the results of the Drug Efficacy Study Implementation (DESI) program (which indicated a lack of efficacy). The new formulation simply replaced the two methamphetamine components with dextroamphetamine and amphetamine components of the same weight (the other two original dextroamphetamine and amphetamine components were preserved), preserved the Obetrol branding, and despite it lacking FDA approval, it still made it onto the market and was marketed and sold by Rexar for many years.
In 1994, Richwood Pharmaceuticals acquired Rexar and began promoting Obetrol as a treatment for ADHD (and later narcolepsy as well), now marketed under the brand name of Adderall, a contraction of the phrase "A.D.D. for All" intended to convey that "it was meant to be kind of an inclusive thing" for marketing purposes.[58] The FDA cited the company for numerous significant CGMP violations related to Obetrol discovered during routine inspections following the acquisition (including issuing a formal warning letter for the violations), then later issued a second formal warning letter to Richwood Pharmaceuticals specifically due to violations of "the new drug and misbranding provisions of the FD&C Act". Following extended discussions with Richwood Pharmaceuticals regarding the resolution of a large number of issues related to the company's numerous violations of FDA regulations, the FDA formally approved the first Obetrol labeling/sNDA revisions in 1996, including a name change to Adderall and a restoration of its status as an approved drug product.[59][60] In 1997 Richwood Pharmaceuticals was acquired by Shire Pharmaceuticals in a $186 million transaction.[58]
Richwood Pharmaceuticals, which later merged with Shire,[58] introduced the Adderall brand in 1996 as an instant-release tablet.[59] In 2006, Shire agreed to sell rights to the Adderall name for the instant-release form of the medication to Duramed Pharmaceuticals.[61] DuraMed Pharmaceuticals was acquired by Teva Pharmaceuticals in 2008 during their acquisition of Barr Pharmaceuticals, including Barr's Duramed division.[62]
The first generic version of Adderall IR was introduced to the market in 2002.[63] Later on, Barr and Shire reached a settlement agreement permitting Barr to offer a generic form of the extended-release drug beginning in April 2009.[63][64]
Commercial formulation
Chemically, Adderall is a mixture of four amphetamine salts; specifically, it is composed of equal parts (by mass) of amphetamine aspartate monohydrate, amphetamine sulfate, dextroamphetamine sulfate, and dextroamphetamine saccharate.[24] This drug mixture has slightly stronger CNS effects than racemic amphetamine due to the higher proportion of dextroamphetamine.[15][18] Adderall is produced as both an immediate-release (IR) and extended-release (XR) formulation.[63][1][24] As of December 2013[update], ten different companies produced generic Adderall IR, while Teva Pharmaceutical Industries, Actavis, and Barr Pharmaceuticals manufactured generic Adderall XR.[63] As of 2013[update], Shire plc, the company that held the original patent for Adderall and Adderall XR, still manufactured brand name Adderall XR, but not Adderall IR.[63]
Comparison to other formulations
Adderall is one of several formulations of pharmaceutical amphetamine, including singular or mixed enantiomers and as an enantiomer prodrug. The table below compares these medications (based on US-approved forms):
| drug | formula | molecular mass [note 2] |
amphetamine base [note 3] |
amphetamine base in equal doses |
doses with equal base content [note 4] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (g/mol) | (percent) | (30 mg dose) | ||||||||
| total | base | total | dextro- | levo- | dextro- | levo- | ||||
| dextroamphetamine sulfate[66][67] | (C9H13N)2•H2SO4 | |||||||||
| amphetamine sulfate[68] | (C9H13N)2•H2SO4 | |||||||||
| Adderall | ||||||||||
| 25% | dextroamphetamine sulfate[66][67] | (C9H13N)2•H2SO4 | ||||||||
| 25% | amphetamine sulfate[68] | (C9H13N)2•H2SO4 | ||||||||
| 25% | dextroamphetamine saccharate[69] | (C9H13N)2•C6H10O8 | ||||||||
| 25% | amphetamine aspartate monohydrate[70] | (C9H13N)•C4H7NO4•H2O | ||||||||
| lisdexamfetamine dimesylate[71] | C15H25N3O•(CH4O3S)2 | |||||||||
| amphetamine base suspension[note 5][72] | C9H13N | |||||||||
Society and culture
Legal status
- In Canada, amphetamines are in Schedule I of the Controlled Drugs and Substances Act, and can only be obtained by prescription.[73]
- In Japan, the use, production, and import of any medicine containing amphetamines is prohibited.[74]
- In South Korea, amphetamines are prohibited.[75]
- In Taiwan, amphetamines including Adderall are Schedule 2 drugs with a minimum five-year prison term for possession.[76] On the contrary, Ritalin can be legally prescribed as a form of treatment of ADHD.[77]
- In Thailand, amphetamines are classified as Type 1 Narcotics.[78]
- In the United Kingdom, amphetamines are regarded as Class B drugs. The maximum penalty for unauthorized possession is five years in prison and an unlimited fine. The maximum penalty for illegal supply is 14 years in prison and an unlimited fine.[79]
- In the United States, amphetamine is a Schedule II prescription drug, classified as a central nervous system (CNS) stimulant.[80]
- Internationally, amphetamine is in Schedule II of the Convention on Psychotropic Substances.[81][82]
Names
A similar combination is also sold under the brand name Mydayis.[25][83] Mydayis contains the amphetamines dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine aspartate monohydrate, and amphetamine sulfate capsule.[25]
Shortages
In February 2023, news organizations began reporting on shortages of Adderall in the United States that have lasted for over five months.[84][85] The US Food and Drug Administration (FDA) first reported the shortage in October 2022.[86] In May 2023, seven months into the shortage, the FDA commissioner stated that "a number of generic drugs are in shortage at any given time because there's not enough profit". He points out that Adderall is a special case because it is a controlled substance and the amount available for prescription is controlled by the Drug Enforcement Administration. He also faults a "tremendous increase in prescribing" due to virtual prescribing and general overprescribing and overdiagnosing, adding that "if only the people that needed these drugs got them, there probably wouldn't be a [stimulant medication] shortage".[87][88] The shortage has continued into 2025.[89][90][91] It has led to the creation and expansion of businesses that outsource the search for Adderall. One company charges $50 per U.S. customer to hire workers in the Philippines or another country to make phone calls to all the pharmacies located near the customer and check whether they have any Adderall. Celebrity endorsements have contributed to the increased demand for Adderall.[92]
Notes
- ↑ The human dopamine transporter contains a high affinity extracellular zinc binding site which, upon zinc binding, inhibits dopamine reuptake and amplifies amphetamine-induced dopamine efflux in vitro.[35][36][37] The human serotonin transporter and norepinephrine transporter do not contain zinc binding sites.[37]
- ↑ For uniformity, molecular masses were calculated using the Lenntech Molecular Weight Calculator[65] and were within 0.01g/mol of published pharmaceutical values.
- ↑ Amphetamine base percentage = molecular massbase / molecular masstotal. Amphetamine base percentage for Adderall = sum of component percentages / 4.
- ↑ dose = (1 / amphetamine base percentage) × scaling factor = (molecular masstotal / molecular massbase) × scaling factor. The values in this column were scaled to a 30 mg dose of dextroamphetamine sulfate. Due to pharmacological differences between these medications (e.g., differences in the release, absorption, conversion, concentration, differing effects of enantiomers, half-life, etc.), the listed values should not be considered equipotent doses.
- ↑ This product (Dyanavel XR) is an oral suspension (i.e., a drug that is suspended in a liquid and taken by mouth) that contains 2.5 mg/mL of amphetamine base.[72] The amphetamine base contains dextro- to levo-amphetamine in a ratio of 3.2:1,[72] which is approximately the ratio in Adderall. The product uses an ion exchange resin to achieve extended release of the amphetamine base.[72]
- Image legend
Reference notes
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Adderall- dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablet". Teva Pharmaceuticals USA. 8 November 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f22635fe-821d-4cde-aa12-419f8b53db81.
- ↑ 2.0 2.1 "Amphetamine, past and present--a pharmacological and clinical perspective". Journal of Psychopharmacology 27 (6): 479–496. June 2013. doi:10.1177/0269881113482532. PMID 23539642.
- ↑ "Adderall produces increased striatal dopamine release and a prolonged time course compared to amphetamine isomers". Psychopharmacology 191 (3): 669–677. April 2007. doi:10.1007/s00213-006-0550-9. PMID 17031708.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedLibido - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedMalenka_2009 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedErgogenics - ↑ 7.0 7.1 7.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedFDA - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedCochrane - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedStimulant Misuse - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedNHMH_3e-Addiction doses - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedAddiction risk - ↑ Encyclopedia of Psychopharmacology. Berlin, Germany; London, England: Springer. 2010. p. 78. ISBN 9783540686989.
- ↑ "Monoamine transporters and psychostimulant addiction". Biochemical Pharmacology 75 (1): 196–217. January 2008. doi:10.1016/j.bcp.2007.08.003. PMID 17825265.
- ↑ 14.0 14.1 "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, USA: McGraw-Hill Medical. 2009. pp. 154–157. ISBN 9780071481274.
- ↑ 15.00 15.01 15.02 15.03 15.04 15.05 15.06 15.07 15.08 15.09 Miller GM (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". J. Neurochem. 116 (2): 164–76. doi:10.1111/j.1471-4159.2010.07109.x. PMID 21073468.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 "VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse". Ann. N. Y. Acad. Sci. 1216 (1): 86–98. January 2011. doi:10.1111/j.1749-6632.2010.05906.x. PMID 21272013. Bibcode: 2011NYASA1216...86E. "VMAT2 is the CNS vesicular transporter for not only the biogenic amines DA, NE, EPI, 5-HT, and HIS, but likely also for the trace amines TYR, PEA, and thyronamine (THYR) ... [Trace aminergic] neurons in mammalian CNS would be identifiable as neurons expressing VMAT2 for storage, and the biosynthetic enzyme aromatic amino acid decarboxylase (AADC).".
- ↑ Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- ↑ 18.0 18.1 18.2 18.3 18.4 18.5 18.6 Cite error: Invalid
<ref>tag; no text was provided for refs namedWestfall - ↑ 19.0 19.1 19.2 19.3 19.4 "Trace amine-associated receptor 1 is a stereoselective binding site for compounds in the amphetamine class". Bioorg. Med. Chem. 19 (23): 7044–7048. December 2011. doi:10.1016/j.bmc.2011.10.007. PMID 22037049.
- ↑ 20.0 20.1 Explorations in Child Psychiatry. Springer Science & Business Media. 11 November 2013. pp. 93–94. ISBN 9781468421279. https://books.google.com/books?id=Ob7eBwAAQBAJ. Retrieved 28 April 2015.
- ↑ 21.0 21.1 Arnold LE (2000). "Methyiphenidate vs. Amphetamine: Comparative review". Journal of Attention Disorders 3 (4): 200–211. doi:10.1177/108705470000300403.
- ↑ "Top 300 of 2023". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Dextroamphetamine; Dextroamphetamine Saccharate; Amphetamine; Amphetamine Aspartate Drug Usage Statistics, United States, 2014 - 2023". https://clincalc.com/DrugStats/Drugs/DextroamphetamineDextroamphetamineSaccharateAmphetamineAmphetamineAspartate.
- ↑ 24.00 24.01 24.02 24.03 24.04 24.05 24.06 24.07 24.08 24.09 Cite error: Invalid
<ref>tag; no text was provided for refs namedAdderall XR FDA label - ↑ 25.0 25.1 25.2 25.3 25.4 25.5 25.6 Cite error: Invalid
<ref>tag; no text was provided for refs namedMydayis FDA label - ↑ "Generic Adderall Availability". https://www.drugs.com/availability/generic-adderall.html.
- ↑ "Generic Adderall XR Availability". https://www.drugs.com/availability/generic-adderall-xr.html.
- ↑ "Mydayis (mixed-salts of a single-entity amphetamine product) – First-time generic". https://professionals.optumrx.com/content/dam/optum3/professional-optumrx/news/rxnews/new-generics/newgenerics_mydayis_2023-1012.pdf.
- ↑ 29.0 29.1 "Do pro sports leagues have an Adderall problem?". 27 November 2012. https://www.usatoday.com/story/sports/nfl/2012/11/27/adderall-in-pro-sports/1730431/.
- ↑ "Commonly Abused Prescription Drugs Chart". National Institute on Drug Abuse. http://www.drugabuse.gov/drugs-abuse/commonly-abused-drugs/commonly-abused-prescription-drugs-chart.
- ↑ "Stimulant ADHD Medications – Methylphenidate and Amphetamines". National Institute on Drug Abuse. http://www.drugabuse.gov/publications/infofacts/stimulant-adhd-medications-methylphenidate-amphetamines.
- ↑ 32.0 32.1 32.2 "Mitigating risks of students use of study drugs through understanding motivations for use and applying harm reduction theory: a literature review". Harm Reduction Journal 14 (1). 6 October 2017. doi:10.1186/s12954-017-0194-6. ISSN 1477-7517. PMID 28985738.
- ↑ 33.0 33.1 "National Institute on Drug Abuse. 2009. Stimulant ADHD Medications – Methylphenidate and Amphetamines". National Institute on Drug Abuse. https://nida.nih.gov/publications/research-reports/misuse-prescription-drugs/overview.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedFMO - ↑ "SPECT and PET of the dopamine transporter in attention-deficit/hyperactivity disorder". Expert Rev. Neurother. 8 (4): 611–625. April 2008. doi:10.1586/14737175.8.4.611. PMID 18416663. "Zinc binds at ... extracellular sites of the DAT [103], serving as a DAT inhibitor. In this context, controlled double-blind studies in children are of interest, which showed positive effects of zinc [supplementation] on symptoms of ADHD [105,106]. It should be stated that at this time [supplementation] with zinc is not integrated in any ADHD treatment algorithm.".
- ↑ "How addictive drugs disrupt presynaptic dopamine neurotransmission". Neuron 69 (4): 628–649. February 2011. doi:10.1016/j.neuron.2011.02.010. PMID 21338876. "They did not confirm the predicted straightforward relationship between uptake and release, but rather that some compounds including AMPH were better releasers than substrates for uptake. Zinc, moreover, stimulates efflux of intracellular [3H]DA despite its concomitant inhibition of uptake (Scholze et al., 2002).".
- ↑ 37.0 37.1 "The role of zinc ions in reverse transport mediated by monoamine transporters". J. Biol. Chem. 277 (24): 21505–21513. June 2002. doi:10.1074/jbc.M112265200. PMID 11940571. "The human dopamine transporter (hDAT) contains an endogenous high affinity Zn2+ binding site with three coordinating residues on its extracellular face (His193, His375, and Glu396). ... Although Zn2+ inhibited uptake, Zn2+ facilitated [3H]MPP+ release induced by amphetamine, MPP+, or K+-induced depolarization specifically at hDAT but not at the human serotonin and the norepinephrine transporter (hNET). ... Surprisingly, this amphetamine-elicited efflux was markedly enhanced, rather than inhibited, by the addition of 10 μM Zn2+ to the superfusion buffer (Fig. 2 A, open squares). We stress that Zn2+ per se did not affect basal efflux (Fig. 2 A). ... In many brain regions, Zn2+ is stored in synaptic vesicles and co-released together with glutamate; under basal conditions, the extracellular levels of Zn2+ are low (~10 nM; see Refs. 39, 40). Upon neuronal stimulation, however, Zn2+ is co-released with the neurotransmitters and, consequently, the free Zn2+ concentration may transiently reach values that range from 10–20 μM (10) up to 300 μM (11). The concentrations of Zn2+ shown in this study, required for the stimulation of dopamine release (as well as inhibition of uptake), covered this physiologically relevant range, with maximum stimulation occurring at 3–30 μM. It is therefore conceivable that the action of Zn2+ on hDAT does not merely reflect a biochemical peculiarity but that it is physiologically relevant. ... Thus, when Zn2+ is co-released with glutamate, it may greatly augment the efflux of dopamine.".
- ↑ "Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses". J. Am. Acad. Child Adolesc. Psychiatry 51 (10): 1003–1019.e20. October 2012. doi:10.1016/j.jaac.2012.08.015. PMID 23021477. "Although we did not find a sufficient number of studies suitable for a meta-analysis of PEA and ADHD, three studies20,57,58 confirmed that urinary levels of PEA were significantly lower in patients with ADHD compared with controls. ... Administration of D-amphetamine and methylphenidate resulted in a markedly increased urinary excretion of PEA,20,60 suggesting that ADHD treatments normalize PEA levels. ... Similarly, urinary biogenic trace amine PEA levels could be a biomarker for the diagnosis of ADHD,20,57,58 for treatment efficacy,20,60 and associated with symptoms of inattentivenesss.59 ... With regard to zinc supplementation, a placebo controlled trial reported that doses up to 30 mg/day of zinc were safe for at least 8 weeks, but the clinical effect was equivocal except for the finding of a 37% reduction in amphetamine optimal dose with 30 mg per day of zinc.110".
- ↑ "A systematic review of combination therapy with stimulants and atomoxetine for attention-deficit/hyperactivity disorder, including patient characteristics, treatment strategies, effectiveness, and tolerability". J Child Adolesc Psychopharmacol 23 (3): 179–193. April 2013. doi:10.1089/cap.2012.0093. PMID 23560600.
- ↑ "ADHD: current and future therapeutics". Behavioral Neuroscience of Attention Deficit Hyperactivity Disorder and Its Treatment. Current Topics in Behavioral Neurosciences. 9. 2012. pp. 361–390. doi:10.1007/7854_2011_125. ISBN 978-3-642-24611-1. "Adjunctive therapy with DL-methylphenidate in atomoxetine partial responders has been successful (Wilens et al. 2009), but this also increases the rates of insomnia, irritability and loss of appetite (Hammerness et al. 2009). This combination therapy has not included amphetamine because blockade of NET by atomoxetine prevents entry of amphetamine into presynaptic noradrenergic terminals (Sofuoglu et al. 2009)."
- ↑ "Atomoxetine attenuates dextroamphetamine effects in humans". Am J Drug Alcohol Abuse 35 (6): 412–416. 2009. doi:10.3109/00952990903383961. PMID 20014909.
- ↑ "Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter". Drug and Alcohol Dependence 147: 1–19. February 2015. doi:10.1016/j.drugalcdep.2014.12.005. PMID 25548026.
- ↑ 43.0 43.1 Forsyth, Andrea N (22 May 2012). Synthesis and Biological Evaluation of Rigid Analogues of Methamphetamines. https://scholarworks.uno.edu/td/1436/. Retrieved 4 November 2024.
- ↑ 44.0 44.1 "Dopamine-releasing agents". Dopamine Transporters: Chemistry, Biology and Pharmacology. Hoboken [NJ]: Wiley. July 2008. pp. 305–320. ISBN 978-0-470-11790-3. OCLC 181862653. https://bitnest.netfirms.com/external/Books/Dopamine-releasing-agents_c11.pdf.
- ↑ 45.0 45.1 45.2 "Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin". Synapse 39 (1): 32–41. January 2001. doi:10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. PMID 11071707.
- ↑ "Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive 'bath salts' products". Neuropsychopharmacology 38 (4): 552–562. 2013. doi:10.1038/npp.2012.204. PMID 23072836.
- ↑ "The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue". Neuropsychopharmacology 37 (5): 1192–1203. 2012. doi:10.1038/npp.2011.304. PMID 22169943.
- ↑ "Monoamine transporters and psychostimulant drugs". Eur J Pharmacol 479 (1–3): 23–40. October 2003. doi:10.1016/j.ejphar.2003.08.054. PMID 14612135.
- ↑ "Therapeutic potential of monoamine transporter substrates". Current Topics in Medicinal Chemistry 6 (17): 1845–1859. 2006. doi:10.2174/156802606778249766. PMID 17017961. https://zenodo.org/record/1235860.
- ↑ "Striatal dopamine neurotransmission: regulation of release and uptake". Basal Ganglia 6 (3): 123–148. August 2016. doi:10.1016/j.baga.2016.02.001. PMID 27141430. "Despite the challenges in determining synaptic vesicle pH, the proton gradient across the vesicle membrane is of fundamental importance for its function. Exposure of isolated catecholamine vesicles to protonophores collapses the pH gradient and rapidly redistributes transmitter from inside to outside the vesicle. ... Amphetamine and its derivatives like methamphetamine are weak base compounds that are the only widely used class of drugs known to elicit transmitter release by a non-exocytic mechanism. As substrates for both DAT and VMAT, amphetamines can be taken up to the cytosol and then sequestered in vesicles, where they act to collapse the vesicular pH gradient.".
- ↑ "Electrophysiological effects of trace amines on mesencephalic dopaminergic neurons". Front. Syst. Neurosci. 5: 56. July 2011. doi:10.3389/fnsys.2011.00056. PMID 21772817. "Three important new aspects of TAs action have recently emerged: (a) inhibition of firing due to increased release of dopamine; (b) reduction of D2 and GABAB receptor-mediated inhibitory responses (excitatory effects due to disinhibition); and (c) a direct TA1 receptor-mediated activation of GIRK channels which produce cell membrane hyperpolarization.".
- ↑ "TAAR1". GenAtlas. University of Paris. 28 January 2012. http://genatlas.medecine.univ-paris5.fr/fiche.php?symbol=TAAR1. Retrieved 29 May 2014. " • tonically activates inwardly rectifying K(+) channels, which reduces the basal firing frequency of dopamine (DA) neurons of the ventral tegmental area (VTA)"
- ↑ "Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons". Neuron 83 (2): 404–416. July 2014. doi:10.1016/j.neuron.2014.05.043. PMID 25033183. "AMPH also increases intracellular calcium (Gnegy et al., 2004) that is associated with calmodulin/CamKII activation (Wei et al., 2007) and modulation and trafficking of the DAT (Fog et al., 2006; Sakrikar et al., 2012). ... For example, AMPH increases extracellular glutamate in various brain regions including the striatum, VTA and NAc (Del Arco et al., 1999; Kim et al., 1981; Mora and Porras, 1993; Xue et al., 1996), but it has not been established whether this change can be explained by increased synaptic release or by reduced clearance of glutamate. ... DHK-sensitive, EAAT2 uptake was not altered by AMPH (Figure 1A). The remaining glutamate transport in these midbrain cultures is likely mediated by EAAT3 and this component was significantly decreased by AMPH".
- ↑ "Mechanisms of dopamine transporter regulation in normal and disease states". Trends Pharmacol. Sci. 34 (9): 489–496. September 2013. doi:10.1016/j.tips.2013.07.005. PMID 23968642. "AMPH and METH also stimulate DA efflux, which is thought to be a crucial element in their addictive properties [80], although the mechanisms do not appear to be identical for each drug [81]. These processes are PKCβ– and CaMK–dependent [72, 82], and PKCβ knock-out mice display decreased AMPH-induced efflux that correlates with reduced AMPH-induced locomotion [72].".
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedcognition enhancers - ↑ "Amphetamine: Biomolecular Interactions and Pathways". National Center for Biotechnology Information. https://pubchem.ncbi.nlm.nih.gov/compound/3007.
- ↑ "Comparative effects of d-amphetamine, l-amphetamine, and methylphenidate on mood in man". Psychopharmacology 53 (1): 1–12. June 1977. doi:10.1007/bf00426687. PMID 407607.
- ↑ 58.0 58.1 58.2 "The Selling of Attention Deficit Disorder". The New York Times. 14 December 2013. ISSN 0362-4331. https://www.nytimes.com/2013/12/15/health/the-selling-of-attention-deficit-disorder.html.
- ↑ 59.0 59.1 "Adderall FDA Review". U.S. Food and Drug Administration (FDA). https://www.accessdata.fda.gov/drugsatfda_docs/nda/96/11522S010_Adderall.pdf.
- ↑ "REGULATORY NEWS: Richwood's Adderall". Health News Daily. 22 February 1996. http://www.elsevierbi.com/Publications/Health-News-Daily/1996/2/22/REGULATORY-NEWS-Richwoods-Adderall.
- ↑ "August 2006 News Archives: Barr and Shire Sign Three Agreements". GenericsWeb. http://www.genericsweb.com/news/generics_industry_news_archive/August2006.html. "WOODCLIFF LAKE, N.J., Aug. 14 /PRNewswire-FirstCall/ – Barr Pharmaceuticals, Inc. today announced that its subsidiary Duramed Pharmaceuticals, Inc. and Shire plc have signed a Product Acquisition Agreement for ADDERALL(R) (immediate-release mixed amphetamine salts) tablets and a Product Development Agreement for six proprietary products, and that its subsidiary Barr Laboratories, Inc. (Barr) has signed a Settlement and License Agreement relating to the resolution of two pending patent cases involving Shire's ADDERALL XR(R) ..."
- ↑ "Teva Completes Acquisition of Barr". Drugs.com. https://www.drugs.com/news/teva-completes-acquisition-barr-15356.html.
- ↑ 63.0 63.1 63.2 63.3 63.4 "National Drug Code Amphetamine Search Results". U.S. Food and Drug Administration (FDA). http://www.accessdata.fda.gov/scripts/cder/ndc/results.cfm?beginrow=1&numberperpage=160&searchfield=amphetamine&searchtype=ActiveIngredient&OrderBy=ProprietaryName.
- ↑ "Teva sells 1st generic of Adderall XL in US". Forbes Magazine. Associated Press. 2 April 2009. https://www.forbes.com/feeds/ap/2009/04/02/ap6248888.html.
- ↑ "Molecular Weight Calculator". Lenntech. http://www.lenntech.com/calculators/molecular/molecular-weight-calculator.htm. Retrieved 19 August 2015.
- ↑ 66.0 66.1 "Dextroamphetamine Sulfate USP". Mallinckrodt Pharmaceuticals. March 2014. http://www2.mallinckrodt.com/WorkArea/DownloadAsset.aspx?id=2147491568. Retrieved 19 August 2015.
- ↑ 67.0 67.1 "D-amphetamine sulfate". Tocris. 2015. http://www.tocris.com/dispprod.php?ItemId=5305#.VXpspvlViko. Retrieved 19 August 2015.
- ↑ 68.0 68.1 "Amphetamine Sulfate USP". Mallinckrodt Pharmaceuticals. March 2014. http://www2.mallinckrodt.com/WorkArea/DownloadAsset.aspx?id=2147491599. Retrieved 19 August 2015.
- ↑ "Dextroamphetamine Saccharate". Mallinckrodt Pharmaceuticals. March 2014. http://www2.mallinckrodt.com/WorkArea/DownloadAsset.aspx?id=2147491576. Retrieved 19 August 2015.
- ↑ "Amphetamine Aspartate". Mallinckrodt Pharmaceuticals. March 2014. http://www2.mallinckrodt.com/WorkArea/DownloadAsset.aspx?id=2147491591. Retrieved 19 August 2015.
- ↑ "Vyvanse Prescribing Information". Shire US Inc.. May 2017. pp. 17–21. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021977s045,208510s001lbl.pdf. Retrieved 10 July 2017.
- ↑ "Controlled Drugs and Substances Act (S.C. 1996, c. 19)". 25 April 2017. http://laws-lois.justice.gc.ca/eng/acts/C-38.8/.
- ↑ "Importing or Bringing Medication into Japan for Personal Use". Japan Ministry of Health, Labour and Welfare. http://www.mhlw.go.jp/english/topics/import/.
- ↑ P. (25 May 2012). "Moving to Korea brings medical, social changes". The Korean Times. https://www.koreatimes.co.kr/www/news/nation/2012/10/319_111757.html.
- ↑ "Caught in the Dragnet". 2 August 2002. http://www.taipeitimes.com/News/feat/archives/2002/08/11/0000159895/4.
- ↑ "吃「聰明藥」反而變笨?小孩能吃嗎?利他能副作用公開". 28 June 2023. https://www.commonhealth.com.tw/article/88370.
- ↑ "Thailand Law". Government of Thailand. http://narcotic.fda.moph.go.th/faq/upload/Thai%20Narcotic%20Act%202012.doc._37ef.pdf.
- ↑ "Class A, B and C drugs". Home Office, Government of the United Kingdom. http://www.homeoffice.gov.uk/drugs/drugs-law/Class-a-b-c/.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs named:USAS2 - ↑ United Nations Office on Drugs and Crime (2007). Preventing Amphetamine-type Stimulant Use Among Young People: A Policy and Programming Guide. New York: United Nations. ISBN 978-92-1-148223-2. http://www.unodc.org/pdf/youthnet/ATS.pdf. Retrieved 7 February 2012.
- ↑ International Narcotics Control Board. "List of psychotropic substances under international control". Vienna: United Nations. http://www.incb.org/pdf/e/list/green.pdf.
- ↑ "Shire launches new ADHD drug Mydayis as it weighs a neuroscience exit". 28 August 2017. https://www.fiercepharma.com/marketing/shire-launches-new-adhd-drug-mydayis-as-it-weighs-a-neuroscience-exit.
- ↑ "The ADHD medication shortage is getting worse. What went wrong?—Neither drugmakers nor the DEA anticipated a sharp rise in ADHD diagnoses during the pandemic. Now an entire class of medications may be in short supply.". NBC. 6 February 2023. https://www.nbcnews.com/health/health-news/adderall-shortage-adhd-drugs-affected-will-end-rcna66766.
- ↑ "Adderall shortage raises questions about widespread dependency on the drug". PBS Newshour. 25 November 2022. https://www.pbs.org/newshour/show/adderall-shortage-raises-questions-about-widespread-dependency-on-the-drug.
- ↑ "Where's the Urgency on the Adderall Shortage?". Intelligencer. 27 March 2023. https://nymag.com/intelligencer/2023/03/wheres-the-urgency-on-the-adderall-shortage.html.
- ↑ "'Exciting Time': FDA Commissioner Talks AI and Misinformation". WebMD. 31 May 2023. https://www.webmd.com/a-to-z-guides/news/20230530/fda-commissioner-talks-ai-and-misinformation.
- ↑ "FDA Commissioner Blames Adderall Shortage on Stimulant Overuse, Telehealth, Generics". Anni Layne Rodgers via Additidemag. 2 June 2023. https://www.additudemag.com/adderall-shortage-stimulant-use-telehealth-califf/.
- ↑ "ADHD medication scarcity adds to questions on US supply chain". 10 October 2025. https://pharmaphorum.com/sales-marketing/adhd-medication-scarcity-adds-questions-us-supply-chain.
- ↑ Smith, Jane (15 May 2024). "Ongoing Adderall Shortage Affects Millions". Health News. https://www.healthnews.com/adderall-shortage-2024.
- ↑ "FDA Update on Adderall Shortage". 20 June 2024. https://www.fda.gov/drugs/drug-safety-and-availability/adderall-shortage-2024-update.
- ↑ Maiberg, Emanuel (11 June 2024). "Americans Are Hiring People in the Philippines to Help Them Find Adderall". https://www.404media.co/people-in-the-philippines-are-calling-us-pharmacies-to-help-americans-find-adderall/.
Template:Wakefulness-promoting agents
 |