Biology:Cyclin-dependent kinase 4
 Generic protein structure example |
Cyclin-dependent kinase 4 also known as cell division protein kinase 4 is an enzyme that in humans is encoded by the CDK4 gene. CDK4 is a member of the cyclin-dependent kinase family.
Function
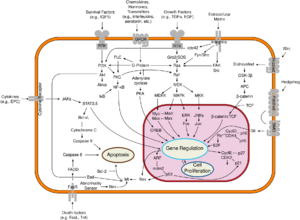
The protein encoded by this gene is a member of the Ser/Thr protein kinase family. This protein is highly similar to the gene products of S. cerevisiae cdc28 and S. pombe cdc2. It is a catalytic subunit of the protein kinase complex that is important for cell cycle G1 phase progression. The activity of this kinase is restricted to the G1-S phase, which is controlled by the regulatory subunits D-type cyclins and CDK inhibitor p16INK4a. This kinase was shown to be responsible for the phosphorylation of retinoblastoma gene product (Rb).[1] Ser/Thr-kinase component of cyclin D-CDK4 (DC) complexes that phosphorylate and inhibit members of the retinoblastoma (RB) protein family including RB1 and regulate the cell-cycle during G1/S transition. Phosphorylation of RB1 allows dissociation of the transcription factor E2F from the RB/E2F complexes and the subsequent transcription of E2F target genes which are responsible for the progression through the G1 phase. Hypophosphorylates RB1 in early G1 phase. Cyclin D-CDK4 complexes are major integrators of various mitogenic and antimitogenic signals. Also phosphorylates SMAD3 in a cell-cycle-dependent manner and represses its transcriptional activity. Component of the ternary complex, cyclin D/CDK4/CDKN1B, required for nuclear translocation and activity of the cyclin D-CDK4 complex.[2]
Clinical significance
Mutations in this gene as well as in its related proteins including D-type cyclins, p16(INK4a), CDKN2A and Rb were all found to be associated with tumorigenesis of a variety of cancers. One specific point mutation of CDK4 (R24C) was first identified in melanoma patients. This mutation was introduced also in animal models and its role as a cancer driver oncogene was studied thoroughly. Nowadays, deregulated CDK4 is considered to be a potential therapeutic target in some cancer types and various CDK4 inhibitors are being tested for cancer treatment in clinical trials.[3][4]
Multiple polyadenylation sites of this gene have been reported.[1]
It is regulated by Cyclin D.
Inhibitors
Ribociclib are US FDA approved CDK4 and CDK6 inhibitors for the treatment of estrogen receptor positive/ HER2 negative advanced breast cancer.[5]
See also CDK inhibitor for inhibitors of various CDKs.
Interactions
Cyclin-dependent kinase 4 has been shown to interact with:
- CDC37,[6][7][8][9]
- CDKN1B,[10][11]
- CDKN2B,[12][13]
- CDKN2C,[6][14]
- CEBPA,[15]
- CCND1,[10][11][16][17][18][19]
- CCND3,[10][12][20][21]
- DBNL,[6]
- MyoD,[22][23]
- P16,[6][16][17][19][24][25]
- PCNA,[17][26] and
- SERTAD1.[16][25]
References
- ↑ 1.0 1.1 "Entrez Gene: CDK4 cyclin-dependent kinase 4". https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=1019.
- ↑ "CDK4 - Cyclin-dependent kinase 4 - Homo sapiens (Human) - CDK4 gene & protein". https://www.uniprot.org/uniprot/P11802.
- ↑ Sheppard, K. E.; McArthur, G. A. (2013-10-01). "The Cell-Cycle Regulator CDK4: An Emerging Therapeutic Target in Melanoma" (in en). Clinical Cancer Research 19 (19): 5320–5328. doi:10.1158/1078-0432.CCR-13-0259. ISSN 1078-0432. PMID 24089445.
- ↑ Sobhani; D’Angelo; Pittacolo; Roviello; Miccoli; Corona; Bernocchi; Generali et al. (2019-04-06). "Updates on the CDK4/6 Inhibitory Strategy and Combinations in Breast Cancer" (in en). Cells 8 (4): 321. doi:10.3390/cells8040321. ISSN 2073-4409. PMID 30959874.
- ↑ "Approved Drugs > Ribociclib (Kisqali)". https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm546438.htm.
- ↑ 6.0 6.1 6.2 6.3 "Large-scale mapping of human protein-protein interactions by mass spectrometry". Mol. Syst. Biol. 3 (1): 89. 2007. doi:10.1038/msb4100134. PMID 17353931.
- ↑ "Physical interaction of mammalian CDC37 with CDK4". J. Biol. Chem. 271 (36): 22030–4. 1996. doi:10.1074/jbc.271.36.22030. PMID 8703009.
- ↑ "Interaction between Cdc37 and Cdk4 in human cells". Oncogene 14 (16): 1999–2004. 1997. doi:10.1038/sj.onc.1201036. PMID 9150368.
- ↑ "Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4". Genes Dev. 10 (12): 1491–502. 1996. doi:10.1101/gad.10.12.1491. PMID 8666233.
- ↑ 10.0 10.1 10.2 "Cdk6-cyclin D3 complex evades inhibition by inhibitor proteins and uniquely controls cell's proliferation competence". Oncogene 20 (16): 2000–9. 2001. doi:10.1038/sj.onc.1204375. PMID 11360184.
- ↑ 11.0 11.1 "Down-regulation of p21WAF1/CIP1 or p27Kip1 abrogates antiestrogen-mediated cell cycle arrest in human breast cancer cells". Proc. Natl. Acad. Sci. U.S.A. 97 (16): 9042–6. 2000. doi:10.1073/pnas.160016897. PMID 10908655. Bibcode: 2000PNAS...97.9042C.
- ↑ 12.0 12.1 "Towards a proteome-scale map of the human protein-protein interaction network". Nature 437 (7062): 1173–8. 2005. doi:10.1038/nature04209. PMID 16189514. Bibcode: 2005Natur.437.1173R.
- ↑ "A skeleton of the human protein interactome". Cell 122 (6): 830–2. 2005. doi:10.1016/j.cell.2005.09.006. PMID 16179252.
- ↑ "Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function". Genes Dev. 8 (24): 2939–52. 1994. doi:10.1101/gad.8.24.2939. PMID 8001816.
- ↑ "C/EBPalpha arrests cell proliferation through direct inhibition of Cdk2 and Cdk4". Mol. Cell 8 (4): 817–28. 2001. doi:10.1016/S1097-2765(01)00366-5. PMID 11684017.
- ↑ 16.0 16.1 16.2 "Regulation of CDK4 activity by a novel CDK4-binding protein, p34(SEI-1)". Genes Dev. 13 (22): 3027–33. 1999. doi:10.1101/gad.13.22.3027. PMID 10580009.
- ↑ 17.0 17.1 17.2 "Cell cycle. Dams and sluices". Nature 366 (6456): 634–5. 1993. doi:10.1038/366634a0. PMID 8259207.
- ↑ "Calmodulin is essential for cyclin-dependent kinase 4 (Cdk4) activity and nuclear accumulation of cyclin D1-Cdk4 during G1". J. Biol. Chem. 273 (50): 33279–86. 1998. doi:10.1074/jbc.273.50.33279. PMID 9837900.
- ↑ 19.0 19.1 "Identification of CDK4 sequences involved in cyclin D1 and p16 binding". J. Biol. Chem. 272 (30): 18869–74. 1997. doi:10.1074/jbc.272.30.18869. PMID 9228064.
- ↑ "A novel partner for D-type cyclins: protein kinase A-anchoring protein AKAP95". Biochem. J. 378 (Pt 2): 673–9. 2004. doi:10.1042/BJ20031765. PMID 14641107.
- ↑ "Developmentally regulated expression of cyclin D3 and its potential in vivo interacting proteins during murine gametogenesis". Endocrinology 140 (6): 2790–800. 1999. doi:10.1210/endo.140.6.6756. PMID 10342870.
- ↑ "Direct inhibition of G(1) cdk kinase activity by MyoD promotes myoblast cell cycle withdrawal and terminal differentiation". EMBO J. 18 (24): 6983–93. 1999. doi:10.1093/emboj/18.24.6983. PMID 10601020.
- ↑ "Coupling of the cell cycle and myogenesis through the cyclin D1-dependent interaction of MyoD with cdk4". EMBO J. 18 (4): 926–33. 1999. doi:10.1093/emboj/18.4.926. PMID 10022835.
- ↑ "Inhibition of pRb phosphorylation and cell-cycle progression by a 20-residue peptide derived from p16CDKN2/INK4A". Curr. Biol. 6 (1): 84–91. 1996. doi:10.1016/S0960-9822(02)00425-6. PMID 8805225. https://www.pure.ed.ac.uk/ws/files/12111645/Inhibition_of_pRb_phosphorylation_and_cell_cycle_progression.pdf.
- ↑ 25.0 25.1 "The nuclear protein p34SEI-1 regulates the kinase activity of cyclin-dependent kinase 4 in a concentration-dependent manner". Biochemistry 43 (14): 4394–9. 2004. doi:10.1021/bi035601s. PMID 15065884.
- ↑ "Subunit rearrangement of the cyclin-dependent kinases is associated with cellular transformation". Genes Dev. 7 (8): 1572–83. 1993. doi:10.1101/gad.7.8.1572. PMID 8101826.
Further reading
- Hanks SK (1987). "Homology probing: identification of cDNA clones encoding members of the protein-serine kinase family". Proc. Natl. Acad. Sci. U.S.A. 84 (2): 388–92. doi:10.1073/pnas.84.2.388. PMID 2948189. Bibcode: 1987PNAS...84..388H.
- Hall M; Bates S; Peters G (1995). "Evidence for different modes of action of cyclin-dependent kinase inhibitors: p15 and p16 bind to kinases, p21 and p27 bind to cyclins". Oncogene 11 (8): 1581–8. PMID 7478582.
- Tassan JP; Jaquenoud M; Léopold P et al. (1995). "Identification of human cyclin-dependent kinase 8, a putative protein kinase partner for cyclin C". Proc. Natl. Acad. Sci. U.S.A. 92 (19): 8871–5. doi:10.1073/pnas.92.19.8871. PMID 7568034. Bibcode: 1995PNAS...92.8871T.
- Mitchell EL; White GR; Santibanez-Koref MF et al. (1995). "Mapping of gene loci in the Q13-Q15 region of chromosome 12". Chromosome Res. 3 (4): 261–2. doi:10.1007/BF00713052. PMID 7606365.
- Wölfel T; Hauer M; Schneider J et al. (1995). "A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma". Science 269 (5228): 1281–4. doi:10.1126/science.7652577. PMID 7652577. Bibcode: 1995Sci...269.1281W.
- Hirai H; Roussel MF; Kato JY et al. (1995). "Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6". Mol. Cell. Biol. 15 (5): 2672–81. doi:10.1128/MCB.15.5.2672. PMID 7739547.
- Chan FK; Zhang J; Cheng L et al. (1995). "Identification of human and mouse p19, a novel CDK4 and CDK6 inhibitor with homology to p16ink4". Mol. Cell. Biol. 15 (5): 2682–8. doi:10.1128/MCB.15.5.2682. PMID 7739548.
- Guan KL; Jenkins CW; Li Y et al. (1995). "Growth suppression by p18, a p16INK4/MTS1- and p14INK4B/MTS2-related CDK6 inhibitor, correlates with wild-type pRb function". Genes Dev. 8 (24): 2939–52. doi:10.1101/gad.8.24.2939. PMID 8001816.
- Kato JY; Matsuoka M; Strom DK; Sherr CJ (1994). "Regulation of cyclin D-dependent kinase 4 (cdk4) by cdk4-activating kinase". Mol. Cell. Biol. 14 (4): 2713–21. doi:10.1128/MCB.14.4.2713. PMID 8139570.
- Khatib ZA; Matsushime H; Valentine M et al. (1993). "Coamplification of the CDK4 gene with MDM2 and GLI in human sarcomas". Cancer Res. 53 (22): 5535–41. PMID 8221695.
- Serrano M; Hannon GJ; Beach D (1994). "A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4". Nature 366 (6456): 704–7. doi:10.1038/366704a0. PMID 8259215. Bibcode: 1993Natur.366..704S.
- Demetrick DJ; Zhang H; Beach DH (1994). "Chromosomal mapping of human CDK2, CDK4, and CDK5 cell cycle kinase genes". Cytogenet. Cell Genet. 66 (1): 72–4. doi:10.1159/000133669. PMID 8275715.
- Kato J; Matsushime H; Hiebert SW et al. (1993). "Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4". Genes Dev. 7 (3): 331–42. doi:10.1101/gad.7.3.331. PMID 8449399.
- Zuo L; Weger J; Yang Q et al. (1996). "Germline mutations in the p16INK4a binding domain of CDK4 in familial melanoma". Nat. Genet. 12 (1): 97–9. doi:10.1038/ng0196-97. PMID 8528263.
- Andersson B; Wentland MA; Ricafrente JY et al. (1996). "A "double adaptor" method for improved shotgun library construction". Anal. Biochem. 236 (1): 107–13. doi:10.1006/abio.1996.0138. PMID 8619474.
- Knudsen ES; Wang JY (1996). "Differential regulation of retinoblastoma protein function by specific Cdk phosphorylation sites". J. Biol. Chem. 271 (14): 8313–20. doi:10.1074/jbc.271.14.8313. PMID 8626527.
- Poon RY; Jiang W; Toyoshima H; Hunter T (1996). "Cyclin-dependent kinases are inactivated by a combination of p21 and Thr-14/Tyr-15 phosphorylation after UV-induced DNA damage". J. Biol. Chem. 271 (22): 13283–91. doi:10.1074/jbc.271.22.13283. PMID 8662825.
- Stepanova L; Leng X; Parker SB; Harper JW (1996). "Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4". Genes Dev. 10 (12): 1491–502. doi:10.1101/gad.10.12.1491. PMID 8666233.
- Dai K; Kobayashi R; Beach D (1996). "Physical interaction of mammalian CDC37 with CDK4". J. Biol. Chem. 271 (36): 22030–4. doi:10.1074/jbc.271.36.22030. PMID 8703009.
- Fåhraeus R; Paramio JM; Ball KL et al. (1996). "Inhibition of pRb phosphorylation and cell-cycle progression by a 20-residue peptide derived from p16CDKN2/INK4A". Curr. Biol. 6 (1): 84–91. doi:10.1016/S0960-9822(02)00425-6. PMID 8805225. https://www.pure.ed.ac.uk/ws/files/12111645/Inhibition_of_pRb_phosphorylation_and_cell_cycle_progression.pdf.
External links
- Cyclin-Dependent+Kinase+4 at the US National Library of Medicine Medical Subject Headings (MeSH)
- CDK4 human gene location in the UCSC Genome Browser.
- CDK4 human gene details in the UCSC Genome Browser.
 |


