Chemistry:Oxalyl chloride
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Oxalyl dichloride[1] | |||
| Systematic IUPAC name
Ethanedioyl dichloride[1] | |||
Other names
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| C 2O 2Cl 2 | |||
| Molar mass | 126.92 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Phosgene-like[2] | ||
| Density | 1.4785 g/mL | ||
| Melting point | −16 °C (3 °F; 257 K) | ||
| Boiling point | 63 to 64 °C (145 to 147 °F; 336 to 337 K) at 1.017 bar | ||
| Reacts | |||
Refractive index (nD)
|
1.429 | ||
| Hazards | |||
| Main hazards | Toxic, corrosive, lachrymator[3] | ||
| Safety data sheet | External MSDS | ||
| GHS pictograms |   [3] [3]
| ||
| GHS Signal word | Danger[3] | ||
| H314, H331[3] | |||
| P261, P280, P305+351+338, P310[3] | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related acyl chlorides
|
|||
Related compounds
|
| ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
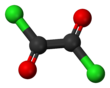
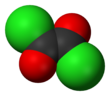
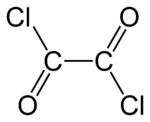
Oxalyl chloride is an organic chemical compound with the formula Cl–C(=O)–C(=O)–Cl. This colorless, sharp-smelling liquid, the diacyl chloride of oxalic acid, is a useful reagent in organic synthesis.[4]
Preparation
Oxalyl chloride was first prepared in 1892 by the French chemist Adrien Fauconnier, who treated diethyl oxalate with phosphorus pentachloride.[5] It can also be prepared by treating oxalic acid with phosphorus pentachloride.[6][7]
Oxalyl chloride is produced commercially from ethylene carbonate. Photochlorination gives the perchloroethylene carbonate C
2Cl
4O
2CO and hydrogen chloride HCl, which is subsequently degraded to oxalyl chloride and phosgene COCl
2:[8]
- C
2H
4O
2CO + 4 Cl
2 → C
2Cl
4O
2CO + 4 HCl - C
2Cl
4O
2CO → C
2O
2Cl
2 + COCl
2
Reactions
As originally determined by Staudinger,[7] oxalyl chloride reacts with water giving off gaseous products only: hydrogen chloride (HCl), carbon dioxide (CO
2), and carbon monoxide (CO).[9]
- (COCl)
2 + H
2O → 2 HCl + CO
2 + CO
Other acyl chlorides hydrolyze with formation of hydrogen chloride and the original carboxylic acid.
Applications in organic synthesis
Oxidation of alcohols
Addition of a primary or secondary alcohol to a solution of oxalyl chloride in DMSO, followed by quenching with an organic base such as triethylamine converts alcohols to the corresponding aldehydes and ketones via the process known as the Swern oxidation.[10][11][12]
Synthesis of acyl chlorides
Oxalyl chloride is mainly used together with a N,N-dimethylformamide catalyst in organic synthesis for the preparation of acyl chlorides from the corresponding carboxylic acids.[citation needed] Like thionyl chloride, the reagent degrades into volatile side products in this application, which simplifies workup. One of the minor byproducts from the N,N-dimethylformamide catalyzed reaction, dimethylcarbamoyl chloride, is a potent carcinogen, stemming from the N,N-dimethylformamide decomposition.[13][14] Relative to thionyl chloride, oxalyl chloride tends to be a milder, more selective reagent. It is also more expensive than thionyl chloride so it tends to be used on a smaller scale.
- 500px
This reaction involves conversion of N,N-dimethylformamide to the imidoyl chloride derivative (chloromethylene(dimethyl)ammonium ion (CH
3)
2N+
=CHCl), akin to the first stage in the Vilsmeier–Haack reaction. The imidoyl chloride is the active chlorinating agent.
Addition of carboxylic acid groups to arenes
Oxalyl chloride reacts with aromatic compounds in the presence of aluminium chloride to give the corresponding acyl chloride in a process known as a Friedel-Crafts acylation.[15][16] The resulting acyl chloride can be hydrolysed to form the corresponding carboxylic acid.
Preparation of oxalate diesters
Like other acyl chlorides, oxalyl chloride reacts with alcohols to give esters:
- 2 R–CH
2–OH + Cl–C(=O)–C(=O)Cl → R–CH
2–O–C(=O)–C(=O)–O–CH
2–R + 2 HCl
A similar reaction occurs with amines to give substituted oxalamides:[17]
- 2 R–NH
2 + ClC(=O)–C(=O)Cl → R–NH–C(=O)–C(=O)–NH–R + 2 HCl
Other
Oxalyl chloride was reportedly used in the first synthesis of dioxane tetraketone (C
4O
6), an oxide of carbon.[18]
Precautions
In March 2000, a Malaysia Airlines Airbus A330-300 was damaged beyond repair after a cargo of prohibited oxalyl chloride (falsely declared as hydroxyquinoline) leaked into the cargo bay.[19] It is toxic by inhalation, although it is over an order of magnitude less acutely toxic than the related compound phosgene.[20]
See also
References
- ↑ 1.0 1.1 Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 797. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ Oxalyl chloride: odor
- ↑ 3.0 3.1 3.2 3.3 3.4 Oxalyl chloride MSDS
- ↑ Salmon, R. (2001). Encyclopedia of Reagents for Organic Synthesis. New York: John Wiley & Sons. doi:10.1002/047084289X.ro015. ISBN 0471936235.
- ↑ Fauconnier, Adrien (1892). "Action du perchlorure de phosphore sur l'oxalate d'éthyle" (in fr). Comptes rendus hebdomadaires des séances de l'Académie des Sciences 114: 122–123. https://babel.hathitrust.org/cgi/pt?id=umn.31951d00008437c;view=1up;seq=132.
- ↑ Vogel, A.; Steffan, G.; Mannes, K.; Trescher, V., "Process for the preparation of oxalyl chloride", DE patent 2840435, issued 1980-03-27, assigned to Bayer
- ↑ 7.0 7.1 Staudinger, H. (1908). "Oxalylchlorid". Berichte der Deutschen Chemischen Gesellschaft 41 (3): 3558–3566. doi:10.1002/cber.19080410335.
- ↑ Pfoertner, Karl-Heinz (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_573.
- ↑ Hansen, Steffen V. F.; Ulven, Trond (2015). "Oxalyl Chloride as a Practical Carbon Monoxide Source for Carbonylation Reactions". Organic Letters 17 (11): 2832–2835. doi:10.1021/acs.orglett.5b01252. PMID 26000869.
- ↑ Dondoni, A.; Perrone, D. (2004). "Synthesis of 1,1-Dimethyl Ethyl-(S)-4-formyl-2,2-dimethyl-3-oxazolidinecarboxylate by Oxidation of the Alcohol". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=v77p0064.; Collective Volume, 10, pp. 320
- ↑ Bishop, R. (1998). "9-Thiabicyclo[3.3.1]nonane-2,6-dione". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv9p0692.; Collective Volume, 9, pp. 692
- ↑ Leopold, E. J. (1990). "Selective hydroboration of a 1,3,7-triene: Homogeraniol". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv7p0258.; Collective Volume, 7, pp. 258
- ↑ Clayden, Jonathan (2005). Organic chemistry (Reprinted(with corrections) ed.). Oxford [u.a.]: Oxford Univ. Press. pp. 296. ISBN 978-0-19-850346-0. https://archive.org/details/organicchemistry00clay_0/page/296.
- ↑ Levin, D. (1997). "Potential Toxicological Concerns Associated with Carboxylic Acid Chlorination and Other Reactions". Organic Process Research & Development (American Chemical Society) 1 (2): 182. doi:10.1021/op970206t.
- ↑ Neubert, M. E.; Fishel, D. L. (1983). "Preparation of 4-Alkyl- and 4-Halobenzoyl Chlorides: 4-Pentylbenzoyl Chloride". Organic Syntheses 61: 8. http://www.orgsyn.org/demo.aspx?prep=cv7p0420.; Collective Volume, 7, pp. 420
- ↑ Sokol, P. E. (1964). "Mesitoic Acid". Organic Syntheses 44: 69. http://www.orgsyn.org/demo.aspx?prep=CV5P0706.; Collective Volume, 5, pp. 706
- ↑ Zou, You-Quan; Zhou, Quan-Quan; Diskin-Posner, Yael; Ben-David, Yehoshoa; Milstein, David (2020). "Synthesis of oxalamides by acceptorless dehydrogenative coupling of ethylene glycol and amines and the reverse hydrogenation catalyzed by ruthenium". Chemical Science 11 (27): 7188–7193. doi:10.1039/D0SC02065F. PMID 34123004.
- ↑ Strazzolini, P.; Gambi, A.; Giumanini, A. G.; Vancik, H. (1998). "The reaction between ethanedioyl (oxalyl) dihalides and Ag2C2O4: a route to Staudinger's elusive ethanedioic (oxalic) acid anhydride". Journal of the Chemical Society, Perkin Transactions 1 1998 (16): 2553–2558. doi:10.1039/a803430c.
- ↑ "Firm told to pay $65 mln for ruining plane". Reuters. 2007-12-06. https://www.reuters.com/article/oddlyEnoughNews/idUSN0620441720071206.
- ↑ Barbee, S.J.; Stone, J.J.; Hilaski, R.J. (January 1995). "Acute Inhalation Toxicology of Oxalyl Chloride" (in en). American Industrial Hygiene Association Journal 56 (1): 74–76. doi:10.1080/15428119591017358. ISSN 0002-8894. PMID 7872205.
 |