Biography:Ernest Rutherford
| module=MonumentsErnest Rutherford memorial, BrightwaterEducationNelson CollegeAlma mater
- Canterbury College (BA, MA, BSc)
- Trinity College, Cambridge (BA)
Known for
- Discovery of the atomic nucleus
- Discovery of radon
- Discovery of the proton
- Theory of the neutron
Spouse(s)
Children1RelativesRalph Fowler (son-in-law)Awards
- FRS (1903)
- Bakerian Medal (1904, 1920)
- Rumford Medal (1904)
- Nobel Prize in Chemistry (1908)
- Elliott Cresson Medal (1910)
- Barnard Medal for Meritorious Service to Science (1910)
- Matteucci Medal (1913)
- Hector Memorial Medal (1916)
- Dalton Medal (1919)
- Copley Medal (1922)
- Franklin Medal (1924)
- Order of Merit (1925)
- RSA Albert Medal (1928)
- IET Faraday Medal (1930)
- Faraday Lectureship Prize (1936)
- Wilhelm Exner Medal (1936)
Title
- Langworthy Professor (1907–19)
- Cavendish Professor of Physics (1919–37)
Scientific careerFields
Institutions
- McGill University
- Victoria University of Manchester
- Cavendish Laboratory
Academic advisors
- Alexander Bickerton[1]
- J. J. Thomson[2]
Doctoral students
- James Chadwick (1921)
- Henry DeWolf Smyth (1923)[1]
- Nazir Ahmed (1925)[3]
- Leslie H. Martin (1926)[1]
- Cecil Powell (1927)[1]
- John Cockcroft (1928)
- Mark Oliphant (1929)[1]
- Norman Feather (1931)[1]
- Ernest Walton (1931)
- Zhang Wenyu (1938)[4][5]
Other notable students
- Kenneth Bainbridge[1]
- Patrick Blackett
- Niels Bohr
- Charles Galton Darwin[1]
- Maria Goeppert Mayer[1]
- Charles Drummond Ellis[1]
- Kazimierz Fajans[1]
- George Gamow[1]
- Hans Geiger
- Otto Hahn[1]
- George de Hevesy[1]
- Ernest Marsden
- Philip Burton Moon[1]
- Henry Moseley
- Frederick Soddy
Signature![]() }}
Ernest Rutherford, Baron Rutherford of Nelson (30 August 1871 – 19 October 1937) was a New Zealand physicist and chemist who was a pioneering researcher in both atomic and nuclear physics. He has been described as "the father of nuclear physics",[6] and "the greatest experimentalist since Michael Faraday".[7] In 1908, he was awarded the Nobel Prize in Chemistry "for his investigations into the disintegration of the elements, and the chemistry of radioactive substances". He was the first Oceanian Nobel laureate, and the first to perform Nobel-awarded work in Canada.
Rutherford's discoveries include the concept of radioactive half-life, the radioactive element radon, and the differentiation and naming of alpha and beta radiation. Together with Thomas Royds, Rutherford is credited with proving that alpha radiation is composed of helium nuclei.[8][9] In 1911, he theorised that atoms have their charge concentrated in a very small nucleus.[10] He arrived at this theory through his discovery and interpretation of Rutherford scattering during the gold foil experiment performed by Hans Geiger and Ernest Marsden. In 1912, he invited Niels Bohr to join his lab, leading to the Bohr model of the atom. In 1917, he performed the first artificially induced nuclear reaction by conducting experiments in which nitrogen nuclei were bombarded with alpha particles. These experiments led him to discover the emission of a subatomic particle that he initially called the "hydrogen atom", but later (more precisely) renamed the proton.[11][12] He is also credited with developing the atomic numbering system alongside Henry Moseley. His other achievements include advancing the fields of radio communications and ultrasound technology.
Rutherford became Director of the Cavendish Laboratory at the University of Cambridge in 1919. Under his leadership, the neutron was discovered by James Chadwick in 1932. In the same year, the first controlled experiment to split the nucleus was performed by John Cockcroft and Ernest Walton, working under his direction. In honour of his scientific advancements, Rutherford was recognised as a baron of the United Kingdom. After his death in 1937, he was buried in Westminster Abbey near Charles Darwin and Isaac Newton. The chemical element rutherfordium (104Rf) was named after him in 1997.
}}
Ernest Rutherford, Baron Rutherford of Nelson (30 August 1871 – 19 October 1937) was a New Zealand physicist and chemist who was a pioneering researcher in both atomic and nuclear physics. He has been described as "the father of nuclear physics",[6] and "the greatest experimentalist since Michael Faraday".[7] In 1908, he was awarded the Nobel Prize in Chemistry "for his investigations into the disintegration of the elements, and the chemistry of radioactive substances". He was the first Oceanian Nobel laureate, and the first to perform Nobel-awarded work in Canada.
Rutherford's discoveries include the concept of radioactive half-life, the radioactive element radon, and the differentiation and naming of alpha and beta radiation. Together with Thomas Royds, Rutherford is credited with proving that alpha radiation is composed of helium nuclei.[8][9] In 1911, he theorised that atoms have their charge concentrated in a very small nucleus.[10] He arrived at this theory through his discovery and interpretation of Rutherford scattering during the gold foil experiment performed by Hans Geiger and Ernest Marsden. In 1912, he invited Niels Bohr to join his lab, leading to the Bohr model of the atom. In 1917, he performed the first artificially induced nuclear reaction by conducting experiments in which nitrogen nuclei were bombarded with alpha particles. These experiments led him to discover the emission of a subatomic particle that he initially called the "hydrogen atom", but later (more precisely) renamed the proton.[11][12] He is also credited with developing the atomic numbering system alongside Henry Moseley. His other achievements include advancing the fields of radio communications and ultrasound technology.
Rutherford became Director of the Cavendish Laboratory at the University of Cambridge in 1919. Under his leadership, the neutron was discovered by James Chadwick in 1932. In the same year, the first controlled experiment to split the nucleus was performed by John Cockcroft and Ernest Walton, working under his direction. In honour of his scientific advancements, Rutherford was recognised as a baron of the United Kingdom. After his death in 1937, he was buried in Westminster Abbey near Charles Darwin and Isaac Newton. The chemical element rutherfordium (104Rf) was named after him in 1997.
Early life and education
Ernest Rutherford was born on 30 August 1871 in Brightwater, New Zealand,[13] the fourth of twelve children of James Rutherford, an immigrant farmer and mechanic from Perth, Scotland, and Martha Thompson, a schoolteacher from Hornchurch, England.[13][14][15] Rutherford's birth certificate was mistakenly written as 'Earnest'. He was known by his family as Ern.[13][15]
When Rutherford was age 5, he moved to Foxhill, New Zealand, and attended Foxhill School. At 11 in 1883, the Rutherford family moved to Havelock, a town in the Marlborough Sounds. The move was made to be closer to the flax mill Rutherford's father developed.[15] Ernest studied at Havelock School.[16]
In 1887, on his second attempt, he won a scholarship to study at Nelson College.[15] On his first examination attempt, he had the highest mark of anyone from Nelson.[17] When he was awarded the scholarship, he had received 580 out of 600 possible marks.[18] After being awarded the scholarship, Havelock School presented him with a five-volume set of books titled The Peoples of the World.[19] He studied at Nelson College between 1887 and 1889, and was head boy in 1889. He also played in the school's rugby team.[15] He was offered a cadetship in government service, but he declined as he still had 15 months of college remaining.[20]
In 1889, after his second attempt, he won a scholarship to study at Canterbury College, University of New Zealand, between 1890 and 1894. He participated in its debating society and the Science Society.[15] At Canterbury, he was awarded a complex B.A. in Latin, English and Maths in 1892, a M.A. in Mathematics and Physical Science in 1893, and a B.Sc. in Chemistry and Geology in 1894.[21][22]
Thereafter, Rutherford invented a new form of a radio receiver, and in 1895 he was awarded an 1851 Research Fellowship from the Royal Commission for the Exhibition of 1851,[23][24] to travel to England for postgraduate study at the Cavendish Laboratory, University of Cambridge.[25] In 1897, he was awarded a B.A. Research Degree and the Coutts-Trotter Studentship from Trinity College, Cambridge.[21]
Career and research

When Rutherford began his studies at Cambridge, he was among the first 'aliens' (those without a Cambridge degree) allowed to do research at the university, and was additionally honoured to study under J. J. Thomson.[2]
With Thomson's encouragement, Rutherford detected radio waves at 0.5 miles (800 m), and briefly held the world record for the distance over which electromagnetic waves could be detected, although when he presented his results at the British Association meeting in 1896, he discovered he had been outdone by Guglielmo Marconi, whose radio waves had sent a message across nearly 10 miles (16 km).[26]
Radioactivity
Again under Thomson's leadership, Rutherford worked on the conductive effects of X-rays on gases, which led to the discovery of the electron, the results first presented by Thomson in 1897.[27][28] Hearing of Henri Becquerel's experience with uranium, Rutherford started to explore its radioactivity, discovering two types that differed from X-rays in their penetrating power. Continuing his research in Canada, in 1899 he coined the terms "alpha ray" and "beta ray" to describe these two distinct types of radiation.[29]
In 1898, Rutherford was accepted to the chair of Macdonald Professor of physics position at McGill University in Montreal, Canada, on Thomson's recommendation.[30] From 1900 to 1903, he was joined at McGill by the young chemist Frederick Soddy (Nobel Prize in Chemistry, 1921) for whom he set the problem of identifying the noble gas emitted by the radioactive element thorium, a substance which was itself radioactive and would coat other substances. Once he had eliminated all the normal chemical reactions, Soddy suggested that it must be one of the inert gases, which they named thoron. This substance was later found to be 220Rn, an isotope of radon.[31][21] They also found another substance they called Thorium X, later identified as 224Rn, and continued to find traces of helium. They also worked with samples of "Uranium X" (protactinium), from William Crookes, and radium, from Marie Curie. Rutherford further investigated thoron in conjunction with R.B. Owens and found that a sample of radioactive material of any size invariably took the same amount of time for half the sample to decay (in this case, 111⁄2 minutes), a phenomenon for which he coined the term "half-life".[31] Rutherford and Soddy published their paper "Law of Radioactive Change" to account for all their experiments. Until then, atoms were assumed to be the indestructible basis of all matter; and although Curie had suggested that radioactivity was an atomic phenomenon, the idea of the atoms of radioactive substances breaking up was a radically new idea. Rutherford and Soddy demonstrated that radioactivity involved the spontaneous disintegration of atoms into other, as yet, unidentified matter.[21]
In 1903, Rutherford considered a type of radiation, discovered (but not named) by French chemist Paul Villard in 1900, as an emission from radium, and realised that this observation must represent something different from his own alpha and beta rays, due to its very much greater penetrating power. Rutherford therefore gave this third type of radiation the name of gamma ray.[29] All three of Rutherford's terms are in standard use today – other types of radioactive decay have since been discovered, but Rutherford's three types are among the most common. In 1904, Rutherford suggested that radioactivity provides a source of energy sufficient to explain the existence of the Sun for the many millions of years required for the slow biological evolution on Earth proposed by biologists such as Charles Darwin. The physicist Lord Kelvin had argued earlier for a much younger Earth, based on the insufficiency of known energy sources, but Rutherford pointed out, at a lecture attended by Kelvin, that radioactivity could solve this problem.[32] Later that year, he was elected as a member to the American Philosophical Society,[33] and in 1907 he returned to Britain to take the chair of physics at the Victoria University of Manchester.[34] He was elected to membership of The Manchester Literary and Philosophical Society 15 October 1907. [35]
In Manchester, Rutherford continued his work with alpha radiation. In conjunction with Hans Geiger, he developed zinc sulfide scintillation screens and ionisation chambers to count alpha particles. By dividing the total charge accumulated on the screen by the number counted, Rutherford determined that the charge on the alpha particle was two.[36][37]: 61 In late 1907, Ernest Rutherford and Thomas Royds allowed alphas to penetrate a very thin window into an evacuated tube. As they sparked the tube into discharge, the spectrum obtained from it changed, as the alphas accumulated in the tube. Eventually, the clear spectrum of helium gas appeared, proving that alphas were at least ionised helium atoms, and probably helium nuclei.[38] In 1910 Rutherford, with Geiger and mathematician Harry Bateman published[39] their classic paper[40]: 94 describing the first analysis of the distribution in time of radioactive emission, a distribution now called the Poisson distribution.
Ernest Rutherford was awarded the 1908 Nobel Prize in Chemistry "for his investigations into the disintegration of the elements, and the chemistry of radioactive substances".[41][21]
Model of the atom
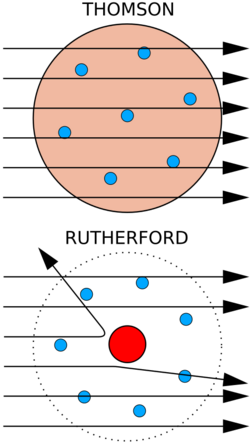
Bottom: Observed results: a small portion of the particles were deflected, indicating a small, concentrated charge. Diagram is not to scale; in reality the nucleus is vastly smaller than the electron shell.
Rutherford continued to make ground-breaking discoveries long after receiving the Nobel prize in 1908.[37]: 63 Under his direction in 1909, Hans Geiger and Ernest Marsden performed the Geiger–Marsden experiment, which demonstrated the nuclear nature of atoms by measuring the deflection of alpha particles passing through a thin gold foil.[42] Rutherford was inspired to ask Geiger and Marsden in this experiment to look for alpha particles with very high deflection angles, which was not expected according to any theory of matter at that time.[43][44] Such deflection angles, although rare, were found. Reflecting on these results in one of his last lectures, Rutherford was quoted as saying: "It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you."[45] It was Rutherford's interpretation of this data that led him to propose the nucleus, a very small, charged region containing much of the atom's mass.[46]
In 1912, Rutherford was joined by Niels Bohr (who postulated that electrons moved in specific orbits about the compact nucleus). Bohr adapted Rutherford's nuclear structure to be consistent with Max Planck's quantum hypothesis. The resulting Bohr model was the basis for quantum mechanical atomic physics of Heisenberg which remains valid today.[21]
Piezoelectricity
During World War I, Rutherford worked on a top-secret project to solve the practical problems of submarine detection. Both Rutherford and Paul Langevin suggested the use of piezoelectricity, and Rutherford successfully developed a device which measured its output. The use of piezoelectricity then became essential to the development of ultrasound as it is known today. The claim that Rutherford developed sonar, however, is a misconception, as subaquatic detection technologies utilise Langevin's transducer.[47][48]
Discovery of the proton
Together with H.G. Moseley, Rutherford developed the atomic numbering system in 1913. Rutherford and Moseley's experiments used cathode rays to bombard various elements with streams of electrons and observed that each element responded in a consistent and distinct manner. Their research was the first to assert that each element could be defined by the properties of its inner structures – an observation that later led to the discovery of the atomic nucleus.[21] This research led Rutherford to theorise that the hydrogen atom (at the time the least massive entity known to bear a positive charge) was a sort of "positive electron" – a component of every atomic element.[49][50]
It was not until 1919 that Rutherford expanded upon his theory of the "positive electron" with a series of experiments beginning shortly before the end of his time at Manchester. He found that nitrogen, and other light elements, ejected a proton, which he called a "hydrogen atom", when hit with α (alpha) particles.[21] In particular, he showed that particles ejected by alpha particles colliding with hydrogen have unit charge and 1/4 the momentum of alpha particles.[51]
Rutherford returned to the Cavendish Laboratory in 1919, succeeding J. J. Thomson as the Cavendish professor and the laboratory's director, posts that he held until his death in 1937.[52] During his tenure, Nobel prizes were awarded to James Chadwick for discovering the neutron (in 1932), John Cockcroft and Ernest Walton for an experiment that was to be known as splitting the atom using a particle accelerator, and Edward Appleton for demonstrating the existence of the ionosphere.
Development of proton and neutron theory
In 1919–1920, Rutherford continued his research on the "hydrogen atom" to confirm that alpha particles break down nitrogen nuclei and to affirm the nature of the products. This result showed Rutherford that hydrogen nuclei were a part of nitrogen nuclei (and by inference, probably other nuclei as well). Such a construction had been suspected for many years, on the basis of atomic weights that were integral multiples of that of hydrogen; see Prout's hypothesis. Hydrogen was known to be the lightest element, and its nuclei presumably the lightest nuclei. Now, because of all these considerations, Rutherford decided that a hydrogen nucleus was possibly a fundamental building block of all nuclei, and also possibly a new fundamental particle as well, since nothing was known to be lighter than that nucleus. Thus, confirming and extending the work of Wilhelm Wien, who in 1898 discovered the proton in streams of ionized gas,[53] in 1920 Rutherford postulated the hydrogen nucleus to be a new particle, which he dubbed the proton.[54]
In 1921, while working with Niels Bohr, Rutherford theorised about the existence of neutrons, (which he had christened in his 1920 Bakerian Lecture), which could somehow compensate for the repelling effect of the positive charges of protons by causing an attractive nuclear force and thus keep the nuclei from flying apart, due to the repulsion between protons. The only alternative to neutrons was the existence of "nuclear electrons", which would counteract some of the proton charges in the nucleus, since by then it was known that nuclei had about twice the mass that could be accounted for if they were simply assembled from hydrogen nuclei (protons). But how these nuclear electrons could be trapped in the nucleus, was a mystery.
In 1932, Rutherford's theory of neutrons was proved by his associate James Chadwick, who recognised neutrons immediately when they were produced by other scientists and later himself, in bombarding beryllium with alpha particles. In 1935, Chadwick was awarded the Nobel Prize in Physics for this discovery.[55]
Induced nuclear reaction and probing the nucleus
In Rutherford's four-part article on the "Collision of α-particles with light atoms" he reported two additional fundamental and far reaching discoveries.[37]: 237 First, he showed that at high angles the scattering of alpha particles from hydrogen differed from the theoretical results he himself published in 1911. These were the first results to probe the interactions that hold a nucleus together. Second, he showed that α-particles colliding with nitrogen nuclei would react rather than simply bounce off. One product of the reaction was the proton; the other product was shown by Patrick Blackett, Rutherford's colleague and former student, to be oxygen:
- 14N + α → 17O + p.
Rutherford therefore recognised "that the nucleus may increase rather than diminish in mass as the result of collisions in which the proton is expelled".[56] Blackett was awarded the Nobel prize in 1948 for his work in perfecting the high-speed cloud chamber apparatus used to make that discovery and many others.[57]
Later years and honours
Rutherford received significant recognition in his home country of New Zealand. In 1901, he earned a DSc from the University of New Zealand.[25] In 1916, he was awarded the Hector Memorial Medal.[58] In 1919 he was awarded the Dalton Medal of the Manchester Literary and Philosophical Society.[59]
In 1925, Rutherford called for the New Zealand Government to support education and research, which led to the formation of the Department of Scientific and Industrial Research (DSIR) in the following year.[60] In 1933, Rutherford was one of the two inaugural recipients of the T. K. Sidey Medal, which was established by the Royal Society of New Zealand as an award for outstanding scientific research.[61][62]
Additionally, Rutherford received a number of awards from the British Crown. He was knighted in 1914.[63] He was appointed to the Order of Merit in the 1925 New Year Honours.[64] Between 1925 and 1930, he served as President of the Royal Society, and later as president of the Academic Assistance Council which helped almost 1,000 university refugees from Germany.[7] In 1931, he was raised to Baron of the United Kingdom under the title Baron Rutherford of Nelson,[65] decorating his coat of arms with a kiwi and a Māori warrior.[66] The title became extinct upon his unexpected death in 1937.
Since 1992, his portrait appears on the New Zealand one hundred-dollar note.
Personal life and death
In the late 1880s Rutherford made his grandmother a wooden potato masher, which is now in the collection of the Royal Society.[67][68]
In 1900, at St Paul's Anglican Church, Papanui in Christchurch, Rutherford married Mary Georgina Newton (1876–1954),[69] to whom he had been engaged before leaving New Zealand.[70][71] They had one daughter, Eileen Mary (1901–1930); she married the physicist Ralph Fowler, and died during the birth of her fourth child. Rutherford's hobbies included golf and motoring.[21]
For some time before his death, Rutherford had a small hernia, which he neglected to have repaired, and it eventually became strangulated, rendering him violently ill. He had an emergency operation in London, but died in Cambridge four days later, on 19 October 1937, at age 66, of what physicians termed "intestinal paralysis".[72] After cremation at Golders Green Crematorium,[72] he was given the high honour of burial in Westminster Abbey, near Isaac Newton, Charles Darwin, and other illustrious British scientists.[21][73]
Legacy

At the opening session of the 1938 Indian Science Congress, which Rutherford had been expected to preside over before his death, astrophysicist James Jeans spoke in his place and deemed him "one of the greatest scientists of all time", saying:
In his flair for the right line of approach to a problem, as well as in the simple directness of his methods of attack, [Rutherford] often reminds us of Faraday, but he had two great advantages which Faraday did not possess, first, exuberant bodily health and energy, and second, the opportunity and capacity to direct a band of enthusiastic co-workers. Great though Faraday's output of work was, it seems to me that to match Rutherford's work in quantity as well as in quality, we must go back to Newton. In some respects he was more fortunate than Newton. Rutherford was ever the happy warrior – happy in his work, happy in its outcome, and happy in its human contacts.[74]
Nuclear physics
Rutherford is known as "the father of nuclear physics" because his research, and work done under him as laboratory director, established the nuclear structure of the atom and the essential nature of radioactive decay as a nuclear process.[6][75][27] Patrick Blackett, a research fellow working under Rutherford, using natural alpha particles, demonstrated induced nuclear transmutation. Later, Rutherford's team, using protons from an accelerator, demonstrated artificially-induced nuclear reactions and transmutation.[76]
Rutherford died too early to see Leó Szilárd's idea of controlled nuclear chain reactions come into being. However, a speech of Rutherford's about his artificially-induced transmutation in lithium, printed in the 12 September 1933 issue of The Times, was reported by Szilárd to have been his inspiration for thinking of the possibility of a controlled energy-producing nuclear chain reaction.[77]
Rutherford's speech touched on the 1932 work of his students John Cockcroft and Ernest Walton in "splitting" lithium into alpha particles by bombardment with protons from a particle accelerator they had constructed. Rutherford realised that the energy released from the split lithium atoms was enormous, but he also realised that the energy needed for the accelerator, and its essential inefficiency in splitting atoms in this fashion, made the project an impossibility as a practical source of energy (accelerator-induced fission of light elements remains too inefficient to be used in this way, even today). Rutherford's speech in part, read:
We might in these processes obtain very much more energy than the proton supplied, but on the average we could not expect to obtain energy in this way. It was a very poor and inefficient way of producing energy, and anyone who looked for a source of power in the transformation of the atoms was talking moonshine. But the subject was scientifically interesting because it gave insight into the atoms.[78][79]
The element rutherfordium, Rf, Z=104, was named in honour of Rutherford in 1997.[80]
In popular culture
Andrew Hodwitz portrays Rutherford in episode 11 of season 13 "Staring Blindly into the Future" (January 13, 2020) of the Canadian television period detective series Murdoch Mysteries.
Publications
Books
- Radio-activity (1904),[81] 2nd ed. (1905), ISBN 978-1-60355-058-1
- Radioactive Transformations (1906), ISBN 978-1-60355-054-3
- (in en) Radioaktive Substanzen und ihre Strahlungen. Cambridge: University press. 1933. https://gutenberg.beic.it/webclient/DeliveryManager?pid=11020002.
- (in de) Radioaktive Substanzen und ihre Strahlungen. Leipzig: Akademische Verlaggesellschaft. 1913. https://gutenberg.beic.it/webclient/DeliveryManager?pid=6739518.
- Radioactive Substances and their Radiations (1913)[82]
- The Electrical Structure of Matter (1926)
- The Artificial Transmutation of the Elements (1933)
- The Newer Alchemy (1937)
Articles
- Ernest Rutherford (1899). "Uranium Radiation and the Electrical conduction Produced by it". Philosophical Magazine 47 (284): 109–163. https://archive.org/details/londonedinburgh5471899lon/page/108/mode/2up.
- Ernest Rutherford (1903). "XV. The Magnetic and Electric Deviation of the easily absorbed Rays from Radium". Philosophical Magazine. 6 5: 177-187. https://archive.org/details/londonedinburgh651903lond/page/176/mode/2up.
- Ernest Rutherford (1906). "The Mass and Velocity of the α particles expelled from Radium and Actinium". Philosophical Magazine. Series 6 12 (70): 348–371. doi:10.1080/14786440609463549. https://zenodo.org/record/1430814.
- Ernest Rutherford; Thomas Royds (1909). "XXI. The nature of the α particle from radioactive substances" (in en). The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science 17 (98): 281–286. doi:10.1080/14786440208636599. ISSN 1941-5982. https://archive.org/details/londonedinburg6171909lond/page/280/mode/2up.
- Ernest Rutherford (1911). "The Scattering of α and β Particles by Matter and the Structure of the Atom". Philosophical Magazine. Series 6 21 (125): 669–688. doi:10.1080/14786440508637080. https://web.mit.edu/8.13/8.13c/references-fall/rutherford/rutherford-scattering-of-alpha-and-beta-particles.pdf.
- Ernest Rutherford (1912). "The origin of β and γ rays from radioactive substances". Philosophical Magazine. Series 6 24 (142): 453–462. doi:10.1080/14786441008637351. https://zenodo.org/record/1430890.
- Ernest Rutherford; John Mitchell Nuttal (1913). "Scattering of α-Particles by Gases". Philosophical Magazine. Series 6 26 (154): 702–712. doi:10.1080/14786441308635014. https://archive.org/details/ClassicalScientificPapersPhysics.
- Ernest Rutherford (1914). "The Structure of the Atom". Philosophical Magazine. Series 6 27 (159): 488–498. doi:10.1080/14786440308635117. http://www.chemteam.info/Chem-History/Rutherford-1914.html.
- Ernest Rutherford (1938). "Forty Years of Physics". Background to Modern Science: Ten Lectures at Cambridge arranged by the History of Science Committee 1936. Cambridge University Press. https://archive.org/details/backgroundtomode032734mbp/page/n85/mode/2up.
- Ernest Rutherford (1913). Radioactive Substances and their Radiations. Cambridge University Press. https://archive.org/details/radioactivesubst00ruthuoft.
- Ernest Rutherford (1936). "Radioactivity and Atomic Structure". Journal of the Chemical Society 1936: 508–516. doi:10.1039/JR9360000508.
- "Disintegration of the Radioactive Elements" Harper's Monthly Magazine, January 1904, pages 279 to 284.
See also
- Bateman equation
- Hydrophone
- Magnetic detector
- Neutron generator
- Royal Society of New Zealand
- Rutherford (unit)
- Rutherfordine
- The Rutherford Journal
- List of presidents of the Royal Society
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 "Ernest Rutherford - Physics Tree". https://academictree.org/physics/peopleinfo.php?pid=13140.
- ↑ 2.0 2.1 "Ernest Rutherford and Frederick Soddy". https://www.aps.org/programs/outreach/history/historicsites/rutherfordsoddy.cfm.
- ↑ "University of the Punjab - Science". http://pu.edu.pk/home/department/55/Department-of-Physics. ""The expedition included Professor James Martin Benade (Professor of Physics at Forman Christian College Lahore) and Dr. Nazir Ahmad (a PhD student of Ernest Rutherford at Cambridge who later on became the First Chairman of Pakistan Atomic Energy Commission in 1956). ""
- ↑ Grodzins, Lee (February 1994). "Obituaries: Zhang Wen-Yu". Physics Today 47 (2): 116. doi:10.1063/1.2808417. "Zhang studied under Ernest Rutherford in the mid-1930s, receiving his degree from Cambridge University in 1938.".
- ↑ Zhang Wenyu (张文裕) (28 March 2018). (in zh)thepaper.com. https://www.thepaper.cn/newsDetail_forward_2047688.
- ↑ 6.0 6.1 "Ernest Rutherford". Michigan State University. https://ehs.msu.edu/lab-clinic/rad/hist-figures/rutherford.html.
- ↑ 7.0 7.1 Badash, Lawrence. "Ernest Rutherford | Accomplishments, Atomic Theory, & Facts | Britannica" (in en). https://www.britannica.com/biography/Ernest-Rutherford.
- ↑ Campbell, John. "Rutherford – A Brief Biography". http://www.rutherford.org.nz/biography.htm.
- ↑ Rutherford, E.; Royds, T. (1908). "Spectrum of the radium emanation". Philosophical Magazine. Series 6 16 (92): 313. doi:10.1080/14786440808636511. https://zenodo.org/record/1430840. Retrieved 28 June 2019.
- ↑ Longair, M. S. (2003). Theoretical concepts in physics: an alternative view of theoretical reasoning in physics. Cambridge University Press. pp. 377–378. ISBN 978-0-521-52878-8. https://books.google.com/books?id=bA9Lp2GH6OEC&pg=PA377. Retrieved 11 May 2020.
- ↑ Rutherford, E. (1919). "Collision of α particles with light atoms. IV. An anomalous effect in nitrogen". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. Series 6 37 (222): 581–587. doi:10.1080/14786440608635919. https://zenodo.org/record/1430800. Retrieved 2 November 2019.
- ↑ Rutherford, E. (1920). "Bakerian Lecture. Nuclear Constitution of Atoms". Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences 97 (686): 374–400. doi:10.1098/rspa.1920.0040. Bibcode: 1920RSPSA..97..374R.
- ↑ 13.0 13.1 13.2 A.H. McLintock (18 September 2007). "Rutherford, Sir Ernest (Baron Rutherford of Nelson, O.M., F.R.S.)". An Encyclopaedia of New Zealand (1966 ed.). Te Ara – The Encyclopedia of New Zealand. ISBN 978-0-478-18451-8. http://www.teara.govt.nz/en/1966/rutherford-sir-ernest/1. Retrieved 2 April 2008.
- ↑ J.L. Heilbron (12 June 2003). Ernest Rutherford And the Explosion of Atoms. Oxford University Press. p. 12. ISBN 0-19-512378-6. https://books.google.com/books?id=_vNW1wg9npgC&pg=PA12. Retrieved 22 February 2016.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 Campbell, John. "Rutherford, Ernest 1871–1937". Dictionary of New Zealand Biography. Ministry for Culture and Heritage. https://teara.govt.nz/en/biographies/3R37.
- ↑ "Local and General News.". Marlborough Express 22 (186): p. 2. 7 October 1886. https://paperspast.natlib.govt.nz/newspapers/MEX18861007.2.8?.
- ↑ "The Marlborough Express. Published Every Evening. Monday, December 28, 1885. Local and General News.". https://paperspast.natlib.govt.nz/newspapers/MEX18851228.2.5.
- ↑ "The Marlborough Express. Published Every Evening Wednesday, January 5, 1887. Local and General News.". https://paperspast.natlib.govt.nz/newspapers/MEX18870105.2.6.
- ↑ "Papers Past | Newspapers | Marlborough Express | 25 January 1887 | Local and General News.". https://paperspast.natlib.govt.nz/newspapers/MEX18870125.2.6.
- ↑ "Papers Past | Newspapers | Marlborough Express | 4 October 1887 | Marlborough Express. Published Every Evening....". https://paperspast.natlib.govt.nz/newspapers/MEX18871004.2.7.
- ↑ 21.00 21.01 21.02 21.03 21.04 21.05 21.06 21.07 21.08 21.09 "Ernest Rutherford Biographical". Nobel Prize Outreach AB. https://www.nobelprize.org/prizes/chemistry/1908/rutherford/biographical/.
- ↑ "Famous Canterbury graduate Ernest Rutherford turns 150" (in en-nz). 27 August 2021. https://www.canterbury.ac.nz/news/2021/famous-canterbury-graduate-ernest-rutherford-turns-150.html.
- ↑ 1851 Royal Commission Archives
- ↑ "Papers Past | Newspapers | Ashburton Guardian | 13 July 1895 | European and Other Foreign Items". https://paperspast.natlib.govt.nz/newspapers/AG18950713.2.9.
- ↑ 25.0 25.1 "Rutherford, Ernest (RTRT895E)". A Cambridge Alumni Database. University of Cambridge. http://venn.lib.cam.ac.uk/cgi-bin/search-2018.pl?sur=&suro=w&fir=&firo=c&cit=&cito=c&c=all&z=all&tex=RTRT895E&sye=&eye=&col=all&maxcount=50.
- ↑ Holmes, Jonathan (13 May 2022). "Marconi's first radio broadcast made 125 years ago". BBC News. https://www.bbc.com/news/uk-england-somerset-61327062.
- ↑ 27.0 27.1 "Know the scientist: Ernest Rutherford" (in en-IN). 17 June 2021. https://www.thehindu.com/children/know-the-scientist-ernest-rutherford/article34837002.ece.
- ↑ Buchwald, Jed Z.; Warwick, Andrew (30 January 2004). Histories of the electron: the birth of microphysics. Cambridge, Mass.: MIT Press. pp. 21–30. ISBN 0262524244. https://books.google.com/books?id=1yqqhlIdCOoC&pg=PA21. Retrieved 27 June 2023.
- ↑ 29.0 29.1 Trenn, Thaddeus J. (1976). "Rutherford on the Alpha-Beta-Gamma Classification of Radioactive Rays". Isis 67 (1): 61–75. doi:10.1086/351545.
- ↑ McKown, Robin (1962). Giant of the Atom, Ernest Rutherford. Julian Messner Inc, New York. p. 57. https://archive.org/details/giantofatomernes00mcko.
- ↑ 31.0 31.1 Kragh, Helge (5 February 2012). "Rutherford, Radioactivity, and the Atomic Nucleus". arXiv:1202.0954 [physics.hist-ph].
- ↑ England, P.; Molnar, P.; Righter, F. (January 2007). "John Perry's neglected critique of Kelvin's age for the Earth: A missed opportunity in geodynamics". GSA Today 17 (1): 4–9. doi:10.1130/GSAT01701A.1. Bibcode: 2007GSAT...17R...4E.
- ↑ "APS Member History". https://search.amphilsoc.org/memhist/search?creator=&title=&subject=&subdiv=&mem=&year=1904&year-max=&dead=&keyword=&smode=advanced.
- ↑ "Ernest Rutherford: Heritage Heroes at The University of Manchester" (in en). https://www.manchester.ac.uk/discover/history-heritage/history/heroes/ernest-rutherford/.
- ↑ Memoirs & Proceedings of Manchester Literary & Philosophical Society. Volume LVl. (1911-12. page lxiv
- ↑ Rutherford, E.; Geiger, Hans (1908-08-27). "The charge and nature of the α-particle" (in en). Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character 81 (546): 162–173. doi:10.1098/rspa.1908.0066. ISSN 0950-1207. Bibcode: 1908RSPSA..81..162R.
- ↑ 37.0 37.1 37.2 Pais, Abraham (2002). Inward bound: of matter and forces in the physical world (Reprint ed.). Oxford: Clarendon Press [u.a.]. ISBN 978-0-19-851997-3.
- ↑ Rutherford, E.; Royds, T. (February 1909). "XXI. The nature of the α particle from radioactive substances". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science 17 (98): 281–286. doi:10.1080/14786440208636599. https://zenodo.org/record/1430648. Retrieved 11 August 2023.
- ↑ Rutherford, E.; Geiger, H.; Bateman, H. (October 1910). "LXXVI. The probability variations in the distribution of α particles". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science 20 (118): 698–707. doi:10.1080/14786441008636955. https://zenodo.org/record/1430880. Retrieved 11 August 2023.
- ↑ Bulmer, M. G. (1979). Principles of Statistics. United Kingdom: Dover Publications.
- ↑ "The Nobel Prize in Chemistry 1908". The Nobel Foundation. https://www.nobelprize.org/prizes/chemistry/1908/summary/.
- ↑ Pestka, Jessica (25 April 2017). "About Rutherford's Gold Foil Experiment" (in en). https://sciencing.com/rutherfords-gold-foil-experiment-4569065.html.
- ↑ Dragovich, Branko. Ernest Rutherford and the Discovery of the Atomic Nucleus. Belgrade: Institute of Physics. http://bsw2011.seenet-mtp.info/pub/bss2011-DragovichB-abs.pdf. Retrieved 27 June 2023.
- ↑ Davidson, Michael W. (March 2014). "Pioneers in Optics: Johann Wilhelm Ritter and Ernest Rutherford". Microscopy Today (Cambridge University Press) 22 (2): 48–51. doi:10.1017/S1551929514000029. https://www.cambridge.org/core/services/aop-cambridge-core/content/view/E8B7456A024C6ED07D4E891F540C8EE2/S1551929514000029a.pdf/pioneers-in-optics-johann-wilhelm-ritter-and-ernest-rutherford.pdf. Retrieved 27 June 2023.
- ↑ The Development of the Theory of Atomic Structure (Rutherford 1936). Reprinted in Background to Modern Science: Ten Lectures at Cambridge arranged by the History of Science Committee 1936
- ↑ Rutherford, E. (1911). "The scattering of α and β particles by matter and the structure of the atom". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. Series 6 21 (125): 669–688. doi:10.1080/14786440508637080. http://www.math.ubc.ca/~cass/rutherford/rutherford688.html. Retrieved 6 October 2012.
- ↑ Katzir, Shaul (20 June 2012). "Who knew piezoelectricity? Rutherford and Langevin on submarine detection and the invention of sonar". Notes and Records of the Royal Society 66 (2): 141–157. doi:10.1098/rsnr.2011.0049. https://royalsocietypublishing.org/doi/epdf/10.1098/rsnr.2011.0049. Retrieved 2 July 2023.
- ↑ Duck, Francis (1 November 2022). "Paul Langevin, U-boats, and ultrasonics". Physics Today 75 (11): 42–48. doi:10.1063/PT.3.5122. Bibcode: 2022PhT....75k..42D.
- ↑ Rutherford, Ernest (1914). "The structure of the atom". Philosophical Magazine 27: 488–498. http://www.ub.edu/hcub/hfq/sites/default/files/ruth1914%285%29.pdf. Retrieved 13 June 2023.
- ↑ Whittaker, Edmund (1989). A History of the Theories of Aether and Electricity. 2. Courier Dover Publications. p. 87. ISBN 0-486-26126-3.
- ↑ Rutherford, Ernest (8 April 2009). "LII. Collision of α particles with light atoms II. Velocity of the hydrogen atom". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 6 37 (222): 562–571. doi:10.1080/14786440608635917. https://www.tandfonline.com/doi/abs/10.1080/14786440608635917?journalCode=tphm17. Retrieved 13 June 2023.
- ↑ "The Cavendish Professorship of Physics". University of Cambridge. http://www.phy.cam.ac.uk/history/cavprof.php. Retrieved 30 November 2013.
- ↑ Wien, W. (1904). "Über positive Elektronen und die Existenz hoher Atomgewichte". Annalen der Physik 318 (4): 669–677. doi:10.1002/andp.18943180404. Bibcode: 1904AnP...318..669W. https://zenodo.org/record/2190505. Retrieved 5 September 2020.
- ↑ Orme Masson (1921). "The Constitution of Atoms". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science 41 (242): 281–285. doi:10.1080/14786442108636219. https://archive.org/details/londonedinburg6411921lond/page/280/mode/2up.
Footnote by Ernest Rutherford: 'At the time of writing this paper in Australia, Professor Orme Masson was not aware that the name "proton" had already been suggested as a suitable name for the unit of mass nearly 1, in terms of oxygen 16, that appears to enter into the nuclear structure of atoms. The question of a suitable name for this unit was discussed at an informal meeting of a number of members of Section A of the British Association at Cardiff this year. The name "baron" suggested by Professor Masson was mentioned, but was considered unsuitable on account of the existing variety of meanings. Finally the name "proton" met with general approval, particularly as it suggests the original term "protyle " given by Prout in his well-known hypothesis that all atoms are built up of hydrogen. The need of a special name for the nuclear unit of mass 1 was drawn attention to by Sir Oliver Lodge at the Sectional meeting, and the writer then suggested the name "proton."' - ↑ "James Chadwick – Facts". Nobel Prize Outreach AB. https://www.nobelprize.org/prizes/physics/1935/chadwick/facts/.
- ↑ Rutherford, Sir Ernest (27 March 1925). "Studies of Atomic Nuclei". Science (The Royal Institution Library of Sciences) 62 (1601): 73–76. doi:10.1126/science.62.1601.209. PMID 17748045. Bibcode: 1925Sci....62..209R. https://archive.org/details/RoyalInstitutionLibraryOfScience-PhysicalScienceVol9. Retrieved 2 October 2023.
- ↑ "Nobel Prize for Physics : Prof. P. M. S. Blackett, F.R.S". Nature 162 (4126): 841. 1948. doi:10.1038/162841b0. Bibcode: 1948Natur.162R.841..
- ↑ "Recipients". Royal Society Te Apārangi. https://www.royalsociety.org.nz/what-we-do/medals-and-awards/hector-medal/recipients-3/.
- ↑ Memoirs And Proceedings Of The Manchester Literary And Philosophical Society Volume 152 2013-14
- ↑ Brewerton, Emma (15 December 2014). "Ernest Rutherford". Ministry for Culture and Heritage. https://nzhistory.govt.nz/people/ernest-rutherford.
- ↑ "Background of the Medal". Royal Society of New Zealand. http://www.royalsociety.org.nz/programmes/awards/sidey-medal/background/.
- ↑ "Recipients". Royal Society of New Zealand. http://www.royalsociety.org.nz/programmes/awards/sidey-medal/recipients/.
- ↑ No. 12647. 27 February 1914. p. 269. https://www.thegazette.co.uk/Edinburgh/issue/12647/page/269
- ↑ No. 14089. 2 January 1925. p. 4. https://www.thegazette.co.uk/Edinburgh/issue/14089/page/4
- ↑ No. 33683. 23 January 1931. p. 533. https://www.thegazette.co.uk/London/issue/33683/page/533
- ↑ "Ernest Rutherford – Biography". https://nzhistory.govt.nz/people/ernest-rutherford.
- ↑ "Ernest Rutherford's potato masher". https://prints.royalsociety.org/products/ernest-rutherfords-potato-masher-rs-8469.
- ↑ "Royal Society Picture Library | Potato masher,Potato masher". https://pictures.royalsociety.org/image-rs-8469.
- ↑ Intergen. "General". https://www.bdmhistoricalrecords.dia.govt.nz/.
- ↑ "Family history in from the cold". 18 March 2009. http://www.stuff.co.nz/the-press/christchurch-life/560019/Family-history-in-from-the-cold.
- ↑ Summerfield, Fiona (9 November 2012). "Historic St Paul's Church in the Christchurch suburb of Papanui is being fully restored". http://anglicantaonga.org.nz/news/tikanga_pakeha/new_lease_of_life_for_historic_chch_church.
- ↑ 72.0 72.1 The Complete Peerage, Volume XIII – Peerage Creations, 1901–1938. St Catherine's Press. 1949. p. 495.
- ↑ Heilbron, J. L. (2003-06-12) (in en). Ernest Rutherford: And the Explosion of Atoms. Oxford University Press. pp. 123–124. ISBN 978-0-19-512378-4. https://books.google.com/books?id=_vNW1wg9npgC&pg=PA123. Retrieved 22 February 2016.
- ↑ "Viceroy Opens The Congress – Sir James Jeans's Address". The Times (Calcutta). 3 January 1938.
- ↑ "Ernest Rutherford: father of nuclear science" (in en). https://media.newzealand.com/en/story-ideas/ernest-rutherford-father-of-nuclear-science/.
- ↑ Giunta, Carmen (2019). "Rutherford and Blackett artificial transmutation". https://web.lemoyne.edu/giunta/classicalcs/ruthblack.html.
- ↑ "September 12, 1933 – Leó Szilárd conceives the idea of the nuclear chain reaction" (in Spanish, English). https://rinconeducativo.org/en/anniversaries/september-12-1933-leo-szilard-conceives-the-idea-of-the-nuclear-chain-reaction/#:~:text=On%20September%2012%2C%201933%2C%20in%20London%2C%20Szil%C3%A1rd%20read,transformation%20of%20atoms%20was%20talking%20about%20%22silly%20alcohol%22..
- ↑ "The British association – breaking down the atom". The Times. 12 September 1933.
- ↑ Rhodes, Richard (1986). The Making of the Atomic Bomb. New York: Simon and Schuster. p. 27. ISBN 0-671-44133-7.
- ↑ Freemantle, Michael (2003). "ACS Article on Rutherfordium". Chemical & Engineering News (American Chemical Society). http://pubs.acs.org/cen/80th/print/rutherfordium.html.
- ↑ "Review of Radio-activity by Ernest Rutherford". The Oxford Magazine (The Proprietors) 23: 347. January 25, 1905. https://books.google.com/books?id=WTPmAAAAMAAJ&pg=PA347. Retrieved 22 March 2023.
- ↑ Carmichael, R. D. (1916). "Book Review: Radioactive Substances and their Radiations". Bulletin of the American Mathematical Society 22 (4): 200. doi:10.1090/s0002-9904-1916-02762-5. https://www.ams.org/journals/bull/1916-22-04/S0002-9904-1916-02762-5/S0002-9904-1916-02762-5.pdf. Retrieved 28 April 2021.
Further reading
- Badash, Lawrence (2008). "Rutherford, Ernest". Oxford Dictionary of National Biography (online ed.). Oxford University Press. doi:10.1093/ref:odnb/35891. (Subscription or UK public library membership required.)
- Cragg, R. H. (1971). "Lord Ernest Rutherford of Nelson (1871–1937)". Royal Institute of Chemistry, Reviews 4 (2): 129. doi:10.1039/RR9710400129.
- Campbell, John. (1999) Rutherford: Scientist Supreme, AAS Publications, Christchurch, ISBN 0-4730-5700-X
- Marsden, E. (1954). "The Rutherford Memorial Lecture, 1954. Rutherford-His Life and Work, 1871–1937". Proceedings of the Royal Society A 226 (1166): 283–305. doi:10.1098/rspa.1954.0254. Bibcode: 1954RSPSA.226..283M.
- Reeves, Richard (2008). A Force of Nature: The Frontier Genius of Ernest Rutherford. New York: W. W. Norton. ISBN 0-393-33369-8
- Rhodes, Richard (1986). The Making of the Atomic Bomb. New York: Simon & Schuster. ISBN 0-671-44133-7
- Wilson, David (1983). Rutherford. Simple Genius, Hodder & Stoughton, ISBN 0-340-23805-4
External links
- Biography and web exhibit American Institute of Physics
- Miss nobel-id as parameter including the Nobel Lecture, 11 December 1908 The Chemical Nature of the Alpha Particles from Radioactive Substances
- The Rutherford Museum
- Rutherford Scientist Supreme
- Newspaper clippings about Ernest Rutherford in the 20th Century Press Archives of the ZBW
- "Ernest Rutherford, 150th anniversary" (in en). https://sebastienfritsch.wixsite.com/ernestrutherford150?lang=en. Well-source site with details on Rutherford's life.
| Academic offices | ||
|---|---|---|
| Preceded by Arthur Schuster |
Langworthy Professor at the University of Manchester 1907–1919 |
Succeeded by Lawrence Bragg |
| Preceded by J. J. Thomson |
Cavendish Professor of Experimental Physics, University of Cambridge 1919-1937 |
Succeeded by Lawrence Bragg |
 |

