Biology:Azotobacter
| Azotobacter | |
|---|---|
| |
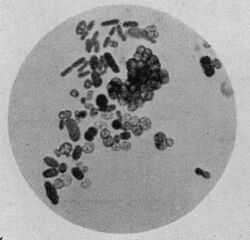
| Azotobacter species cells, stained with Heidenhain's iron hematoxylin, ×1000 | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Pseudomonadota |
| Class: | Gammaproteobacteria |
| Order: | Pseudomonadales |
| Family: | Pseudomonadaceae |
| Genus: | Azotobacter Beijerinck 1901 |
| Species | |
|
Azotobacter agilis | |

Azotobacter is a genus of usually motile, oval or spherical bacteria that form thick-walled cysts (and also has hard crust) and may produce large quantities of capsular slime. They are aerobic, free-living soil microbes that play an important role in the nitrogen cycle in nature, binding atmospheric nitrogen, which is inaccessible to plants, and releasing it in the form of ammonium ions into the soil (nitrogen fixation). In addition to being a model organism for studying diazotrophs, it is used by humans for the production of biofertilizers, food additives, and some biopolymers. The first representative of the genus, Azotobacter chroococcum, was discovered and described in 1901 by Dutch microbiologist and botanist Martinus Beijerinck. Azotobacter species are Gram-negative bacteria found in neutral and alkaline soils,[1][2] in water, and in association with some plants.[3][4]
Biological characteristics
Morphology
Cells of the genus Azotobacter are relatively large for bacteria (2–4 μm in diameter). They are usually oval, but may take various forms from rods to spheres. In microscopic preparations, the cells can be dispersed or form irregular clusters or occasionally chains of varying lengths. In fresh cultures, cells are mobile due to the numerous flagella.[5] Later, the cells lose their mobility, become almost spherical, and produce a thick layer of mucus, forming the cell capsule. The shape of the cell is affected by the amino acid glycine, which is present in the nutrient medium peptone.[6]
Under magnification, the cells show inclusions, some of which are colored. In the early 1900s, the colored inclusions were regarded as "reproductive grains", or gonidia – a kind of embryo cells.[7] However, the granules were later determined to not participate in the cell division.[8] The colored grains are composed of volutin, whereas the colorless inclusions are drops of fat, which act as energy reserves.[9]
Cysts
Cysts of the genus Azotobacter are more resistant to adverse environmental factors than the vegetative cells; in particular, they are twice as resistant to ultraviolet light. They are also resistant to drying, ultrasound, and gamma and solar irradiation, but not to heating.[10]
The formation of cysts is induced by changes in the concentration of nutrients in the medium and addition of some organic substances such as ethanol, n-butanol, or β-hydroxybutyrate. Cysts are rarely formed in liquid media.[11] The formation of cysts is induced by chemical factors and is accompanied by metabolic shifts, changes in catabolism, respiration, and biosynthesis of macromolecules;[12] it is also affected by aldehyde dehydrogenase[13] and the response regulator AlgR.[14]
The cysts of Azotobacter are spherical and consist of the so-called "central body" – a reduced copy of vegetative cells with several vacuoles – and the "two-layer shell". The inner part of the shell is called intine and has a fibrous structure.[15] The outer part has a hexagonal crystalline structure and is called exine.[16] Exine is partially hydrolyzed by trypsin and is resistant to lysozyme, in contrast to the central body.[17] The central body can be isolated in a viable state by some chelation agents.[18] The main constituents of the outer shell are alkylresorcinols composed of long aliphatic chains and aromatic rings. Alkylresorcinols are also found in other bacteria, animals, and plants.[19]
Germination of cysts
A cyst of the genus Azotobacter is the resting form of a vegetative cell; however, whereas usual vegetative cells are reproductive, the cyst of Azotobacter does not serve this purpose and is necessary for surviving adverse environmental factors. Following the resumption of optimal environmental conditions, which include a certain value of pH, temperature, and source of carbon, the cysts germinate, and the newly formed vegetative cells multiply by a simple division. During the germination, the cysts sustain damage and release a large vegetative cell. Microscopically, the first manifestation of spore germination is the gradual decrease in light refractive by cysts, which is detected with phase contrast microscopy. Germination of cysts takes about 4–6 h. During germination, the central body grows and captures the granules of volutin, which were located in the intima (the innermost layer). Then, the exine bursts and the vegetative cell is freed from the exine, which has a characteristic horseshoe shape.[20] This process is accompanied by metabolic changes. Immediately after being supplied with a carbon source, the cysts begin to absorb oxygen and emit carbon dioxide; the rate of this process gradually increases and saturates after four hours. The synthesis of proteins and RNA occurs in parallel, but it intensifies only after five hours after the addition of the carbon source. The synthesis of DNA and nitrogen fixation are initiated 5 hours after the addition of glucose to a nitrogen-free nutrient medium.[21]
Germination of cysts is accompanied by changes in the intima, visible with an electron microscope. The intima consists of carbohydrates, lipids, and proteins and has almost the same volume as the central body. During germination of cysts, the intima hydrolyses and is used by the cell for the synthesis its components.[22]
Physiological properties
Azotobacter respires aerobically, receiving energy from redox reactions, using organic compounds as electron donors, and can use a variety of carbohydrates, alcohols, and salts of organic acids as sources of carbon.
Azotobacter can fix at least 10 μg of nitrogen per gram of glucose consumed. Nitrogen fixation requires molybdenum ions, but they can be partially or completely replaced by vanadium ions. If atmospheric nitrogen is not fixed, the source of nitrogen can alternatively be nitrates, ammonium ions, or amino acids. The optimal pH for the growth and nitrogen fixation is 7.0–7.5, but growth is sustained in the pH range from 4.8 to 8.5.[23] Azotobacter can also grow mixotrophically, in a molecular nitrogen-free medium containing mannose; this growth mode is hydrogen-dependent. Hydrogen is available in the soil, thus this growth mode may occur in nature.[24]
While growing, Azotobacter produces flat, slimy, paste-like colonies with a diameter of 5–10 mm, which may form films in liquid nutrient media. The colonies can be dark-brown, green, or other colors, or may be colorless, depending on the species. The growth is favored at a temperature of 20–30°C.[25]
Bacteria of the genus Azotobacter are also known to form intracellular inclusions of polyhydroxyalkanoates under certain environmental conditions (e.g. lack of elements such as phosphorus, nitrogen, or oxygen combined with an excessive supply of carbon sources).
Pigments
Azotobacter produces pigments. For example, Azotobacter chroococcum forms a dark-brown water-soluble pigment melanin. This process occurs at high levels of metabolism during the fixation of nitrogen, and is thought to protect the nitrogenase system from oxygen.[26] Other Azotobacter species produce pigments from yellow-green to purple colors,[27] including a green pigment which fluoresces with a yellow-green light and a pigment with blue-white fluorescence.[28]
Genome
The nucleotide sequence of chromosomes of Azotobacter vinelandii, strain AvOP, is partially determined. This chromosome is a circular DNA molecule which contains 5,342,073 nucleotide pairs and 5,043 genes, of which 4,988 encode proteins. The fraction of guanine + cytosine pairs is 65 mole percent. The number of chromosomes in the cells and the DNA content increases upon aging, and in the stationary growth phase, cultures may contain more than 100 copies of a chromosome per cell. The original DNA content (one copy) is restored when replanting the culture into a fresh medium.[29] In addition to chromosomal DNA, Azotobacter can contain plasmids.[30]
Distribution
Azotobacter species are ubiquitous in neutral and weakly basic soils, but not acidic soils.[31] They are also found in the Arctic and Antarctic soils, despite the cold climate, short growing season, and relatively low pH values of these soils.[32] In dry soils, Azotobacter can survive in the form of cysts for up to 24 years.[33]
Representatives of the genus Azotobacter are also found in aquatic habitats, including fresh water[34] and brackish marshes.[35] Several members are associated with plants and are found in the rhizosphere, having certain relationships with the plants.[36] Some strains are also found in the cocoons of the earthworm Eisenia fetida.[37]
Nitrogen fixation
Azotobacter species are free-living, nitrogen-fixing bacteria; in contrast to Rhizobium species, they normally fix molecular nitrogen from the atmosphere without symbiotic relations with plants, although some Azotobacter species are associated with plants.[38] Nitrogen fixation is inhibited in the presence of available nitrogen sources, such as ammonium ions and nitrates.[39]
Azotobacter species have a full range of enzymes needed to perform the nitrogen fixation: ferredoxin, hydrogenase, and an important enzyme nitrogenase. The process of nitrogen fixation requires an influx of energy in the form of adenosine triphosphate. Nitrogen fixation is highly sensitive to the presence of oxygen, so Azotobacter developed a special defensive mechanism against oxygen, namely a significant intensification of metabolism that reduces the concentration of oxygen in the cells.[40] Also, a special nitrogenase-protective protein protects nitrogenase and is involved in protecting the cells from oxygen. Mutants not producing this protein are killed by oxygen during nitrogen fixation in the absence of a nitrogen source in the medium.[41] Homocitrate ions play a certain role in the processes of nitrogen fixation by Azotobacter.[42]
Nitrogenase
Nitrogenase is the most important enzyme involved in nitrogen fixation. Azotobacter species have several types of nitrogenase. The basic one is molybdenum-iron nitrogenase.[43] An alternative type contains vanadium; it is independent of molybdenum ions[44][45][46] and is more active than the Mo-Fe nitrogenase at low temperatures. So it can fix nitrogen at temperatures as low as 5 °C, and its low-temperature activity is 10 times higher than that of Mo-Fe nitrogenase.[47] An important role in maturation of Mo-Fe nitrogenase plays the so-called P-cluster.[48] Synthesis of nitrogenase is controlled by the nif genes.[49] Nitrogen fixation is regulated by the enhancer protein NifA and the "sensor" flavoprotein NifL which modulates the activation of gene transcription of nitrogen fixation by redox-dependent switching.[50] This regulatory mechanism, relying on two proteins forming complexes with each other, is uncommon for other systems.[51]
Importance
Nitrogen fixation plays an important role in the nitrogen cycle. Azotobacter also synthesizes some biologically active substances, including some phytohormones such as auxins,[52] thereby stimulating plant growth.[53][54] They also facilitate the mobility of heavy metals in the soil, thus enhancing bioremediation of soil from heavy metals, such as cadmium, mercury and lead.[55] Some kinds of Azotobacter can also biodegrade chlorine-containing aromatic compounds, such as 2,4,6-trichlorophenol, which was previously used as an insecticide, fungicide, and herbicide, but later was found to have mutagenic and carcinogenic effects.[56]
Applications
Owing to their ability to fix molecular nitrogen and therefore increase the soil fertility and stimulate plant growth, Azotobacter species are widely used in agriculture,[57] particularly in nitrogen biofertilizers such as azotobacterin. They are also used in production of alginic acid,[58][59][60] which is applied in medicine as an antacid, in the food industry as an additive to ice cream, puddings, and creams.[61]
Taxonomy

The genus Azotobacter was discovered in 1901 by Dutch microbiologist and botanist Martinus Beijerinck, who was one of the founders of environmental microbiology. He selected and described the species Azotobacter chroococcum – the first aerobic, free-living nitrogen fixer.[62]
In 1909, Lipman described Azotobacter vinelandii, and a year later Template:Btname, which he named in honor of Beijerinck. In 1949, Russian microbiologist Nikolai Krasilnikov identified the species of Template:Btname which was divided in 1981 by Thompson Skerman into two subspecies – Azotobacter nigricans subsp. nigricans and Azotobacter nigricans subsp. achromogenes; in the same year, Thompson and Skerman described Template:Btname. In 1991, Page and Shivprasad reported a microaerophilic and air-tolerant type Template:Btname which was dependent on sodium ions.[63]
Earlier, representatives of the genus were assigned to the family Azotobacteraceae Pribram, 1933, but then were transferred to the family Pseudomonadaceae based on the studies of nucleotide sequences 16S rRNA. In 2004, a phylogenetic study revealed that A. vinelandii belongs to the same clade as the bacterium Pseudomonas aeruginosa,[64] and in 2007 it was suggested that the genera Azotobacter, Azomonas and Pseudomonas are related and might be synonyms.[65]
References
- ↑ Gandora V.; Gupta R. D.; Bhardwaj K. K. R. (1998). "Abundance of Azotobacter in great soil groups of North-West Himalayas". Journal of the Indian Society of Soil Science 46 (3): 379–383. http://cat.inist.fr/?aModele=afficheN&cpsidt=1935573. Retrieved 2010-08-30.
- ↑ Martyniuk S.; Martyniuk M. (2003). "Occurrence of Azotobacter Spp. in Some Polish Soils". Polish Journal of Environmental Studies 12 (3): 371–374. http://www.pjoes.com/pdf/12.3/371-374.pdf. Retrieved 2010-08-30.
- ↑ Tejera N.; Lluch C.; Martínez-Toledo M. V.; González-López J. (2005). "Isolation and characterization of Azotobacter and Azospirillum strains from the sugarcane rhizosphere". Plant and Soil 270 (1–2): 223–232. doi:10.1007/s11104-004-1522-7. http://www.ugr.es/~natejera/TejeraPS2005.pdf. Retrieved 2010-08-30.
- ↑ Kumar R.; Bhatia R.; Kukreja K.; Behl R. K.; Dudeja S. S.; Narula N. (2007). "Establishment of Azotobacter on plant roots: chemotactic response, development and analysis of root exudates of cotton (Gossypium hirsutum L.) and wheat (Triticum aestivum L.)". Journal of Basic Microbiology 47 (5): 436–439. doi:10.1002/jobm.200610285. PMID 17910096.
- ↑ Baillie A.; Hodgkiss W.; Norris J. R. (1962). "Flagellation of Azotobacter spp. as Demonstrated by Electron Microscopy". Journal of Applied Microbiology 25 (1): 116–119. doi:10.1111/j.1365-2672.1962.tb01126.x.
- ↑ Vela G. R.; Rosenthal R. S. (1972). "Effect of Peptone on Azotobacter Morphology". Journal of Bacteriology 111 (1): 260–266. doi:10.1128/JB.111.1.260-266.1972. PMID 4591479.
- ↑ Jones D. H. (1920). "Further Studies on the Growth Cycle of Azotobacter". Journal of Bacteriology 5 (4): 325–341. doi:10.1128/JB.5.4.325-341.1920. PMID 16558880.
- ↑ Lewis I. M. (1941). "The cytology of bacteria". Bacteriological Reviews 5 (3): 181–230. doi:10.1128/MMBR.5.3.181-230.1941. PMID 16350071.
- ↑ Lewis I. M. (1937). "Cell Inclusions and the Life Cycle of Azotobacter". Journal of Bacteriology 34 (2): 191–205. doi:10.1128/JB.34.2.191-205.1937. PMID 16560046.
- ↑ Socolofsky M. D.; Wyss O. (1962). "Resistance of the Azotobacter Cyst". Journal of Bacteriology 84 (1): 119–124. doi:10.1128/JB.84.1.119-124.1962. PMID 13914732.
- ↑ Layne J. S.; Johnson E. J. (1964). "Natural Factors Involved in the Induction of Cyst Formation in Azotobacter". Journal of Bacteriology 87 (3): 684–689. doi:10.1128/JB.87.3.684-689.1964. PMID 14127586.
- ↑ Sadoff H. L. (1975). "Encystment and Germination in Azotobacter vinelandii". Microbiological Reviews 39 (4): 516–539. doi:10.1128/br.39.4.516-539.1975. PMID 1212151.
- ↑ Gama-Castro S.; Núñez C.; Segura D.; Moreno S.; Guzmán J.; Espín G. (2001). "Azotobacter vinelandii Aldehyde Dehydrogenase Regulated by ς54: Role in Alcohol Catabolism and Encystment". Journal of Bacteriology 183 (21): 6169–6174. doi:10.1128/JB.183.21.6169-6174.2001. PMID 11591659.
- ↑ Núñez C.; Moreno S.; Soberón-Chávez G.; Espín G. (1999). "The Azotobacter vinelandii Response Regulator AlgR Is Essential for Cyst Formation". Journal of Bacteriology 181 (1): 141–148. doi:10.1128/JB.181.1.141-148.1999. PMID 9864323.
- ↑ Pope L. M.; Wyss O. (1970). "Outer Layers of the Azotobacter vinelandii Cyst". Journal of Bacteriology 102 (1): 234–239. doi:10.1128/JB.102.1.234-239.1970. PMID 4191240.
- ↑ Page W. J.; Sadoff H. L. (1975). "Relationship between calcium and uroinic acids in the encystment of Azotobacter vinelandii". Journal of Bacteriology 122 (1): 145–151. doi:10.1128/JB.122.1.145-151.1975. PMID 235508.
- ↑ Lin L. P.; Sadoff H. L. (1969). "Preparation and Ultrastructure of the Outer Coats of Azotobacter vinelandii Cysts". Journal of Bacteriology 98 (3): 1335–1341. doi:10.1128/JB.98.3.1335-1341.1969. PMID 4977988.
- ↑ Parker L. T.; Socolofsky M. D. (1968). "Central Body of the Azotobacter Cyst". Journal of Bacteriology 91 (1): 297–303. doi:10.1128/JB.91.1.297-303.1966. PMID 4955249.
- ↑ Funa N.; Ozawa H.; Hirata A.; Horinouchi S. (2006). "Phenolic lipid synthesis by type III polyketide synthases is essential for cyst formation in Azotobacter vinelandii". Proceedings of the National Academy of Sciences of the United States of America 103 (16): 6356–6361. doi:10.1073/pnas.0511227103. PMID 16597676. Bibcode: 2006PNAS..103.6356F.
- ↑ Wyss O.; Neumann M. G.; Socolofsky M. D. (1961). "Development and germination of the Azotobacter cyst". Journal of Biophysical and Biochemical Cytology 10 (4): 555–565. doi:10.1083/jcb.10.4.555. PMID 13787014.
- ↑ Loperfido B.; Sadoff H. L. (1973). "Germination of Azotobacter vinelandii Cysts: Sequence of Macromolecular Synthesis and Nitrogen Fixation". Journal of Bacteriology 113 (2): 841–846. doi:10.1128/JB.113.2.841-846.1973. PMID 4690966.
- ↑ Lin L. P.; Pankratz S.; Sadoff H. L. (1978). "Ultrastructural and physiological changes occurring upon germination and outgrowth of Azotobacter vinelandii cysts". Journal of Bacteriology 135 (2): 641–646. doi:10.1128/JB.135.2.641-646.1978. PMID 681284.
- ↑ George M. Garrity, ed (2005). "Part B: The Gammaproteobacteria". Bergey's Manual of Systematic Bacteriology. The Proteobacteria (2 ed.). New York: Springer. ISBN 978-0-387-95040-2. https://www.springer.com/life+sci/book/978-0-387-95040-2. Retrieved 2017-08-28.
- ↑ Wong T.-Y.; Maier R. J. (1985). "H2-Dependent Mixotrophic Growth of N2-Fixing Azotobacter vinelandii". Journal of Bacteriology 163 (2): 528–533. doi:10.1128/JB.163.2.528-533.1985. PMID 4019408.
- ↑ Workshop on Microbiology. M.. 1979. p. 216.
- ↑ Shivprasad S.; Page W. J. (1989). "Catechol Formation and Melanization by Na+-Dependent Azotobacter chroococcum: a Protective Mechanism for Aeroadaptation?". Applied and Environmental Microbiology 55 (7): 1811–1817. doi:10.1128/AEM.55.7.1811-1817.1989. PMID 16347974. Bibcode: 1989ApEnM..55.1811S.
- ↑ Jensen H. L. (1954). "The Azotobacteriaceae". Bacteriological Reviews 18 (4): 195–214. doi:10.1128/MMBR.18.4.195-214.1954. PMID 13219046.
- ↑ Johnstone D. B. (1955). "Azotobacter Fluorescence". Journal of Bacteriology 69 (4): 481–482. doi:10.1128/JB.69.4.481-482.1955. PMID 14367310.
- ↑ Maldonado R.; Jimenez J.; Casadesus J. (1994). "Changes of Ploidy during the Azotobacter vinelandii Growth Cycle". Journal of Bacteriology 176 (13): 3911–3919. doi:10.1128/jb.176.13.3911-3919.1994. PMID 8021173.
- ↑ Maia M.; Sanchez J. M.; Vela G. R. (1988). "Plasmids of Azotobacter vinelandii". Journal of Bacteriology 170 (4): 1984–1985. doi:10.1128/jb.170.4.1984-1985.1988. PMID 3350795.
- ↑ Yamagata U.; Itano A. (1923). "Physiological Study of Azotobacter chroococcum, beijerinckii and vinelandii types". Journal of Bacteriology 8 (6): 521–531. doi:10.1128/JB.8.6.521-531.1923. PMID 16559016.
- ↑ Boyd W. L.; Boyd J. W. (1962). "Presence of Azotobacter species in Polar Regions". Journal of Bacteriology 83 (2): 429–430. doi:10.1128/JB.83.2.429-430.1962. PMID 16561931.
- ↑ Moreno J.; Gonzalez-Lopez J.; Vela G. R. (1986). "Survival of Azotobacter spp. in Dry Soils". Applied and Environmental Microbiology 51 (1): 123–125. doi:10.1128/AEM.51.1.123-125.1986. PMID 16346962. Bibcode: 1986ApEnM..51..123M.
- ↑ Johnstone D. B. (1967). "Isolation of Azotobacter Insignis From Fresh Water". Ecology 48 (4): 671–672. doi:10.2307/1936516.
- ↑ Dicker H. J.; Smith D. W. (1980). "Enumeration and Relative Importance of Acetylene-Reducing (Nitrogen-Fixing) Bacteria in a Delaware Salt Marsh". Applied and Environmental Microbiology 39 (5): 1019–1025. doi:10.1128/AEM.39.5.1019-1025.1980. PMID 16345564. Bibcode: 1980ApEnM..39.1019D.
- ↑ van Berkum P.; Bohlool B. (1980). "Evaluation of Nitrogen Fixation by Bacteria in Association with Roots of Tropical Grasses". Microbiological Reviews 44 (3): 491–517. doi:10.1128/MMBR.44.3.491-517.1980. PMID 6775181.
- ↑ Zachmann J. E.; Molina J. A. E. (1993). "Presence of Culturable Bacteria in Cocoons of the Earthworm Eisenia fetida". Applied and Environmental Microbiology 59 (6): 1904–1910. doi:10.1128/AEM.59.6.1904-1910.1993. PMID 16348968. Bibcode: 1993ApEnM..59.1904Z.
- ↑ Kass D. L.; Drosdoff M.; Alexander M. (1971). "Nitrogen Fixation by Azotobacter paspali in Association with Bahiagrass (Paspalum notatum)". Soil Science Society of America Journal 35 (2): 286–289. doi:10.2136/sssaj1971.03615995003500020031x. Bibcode: 1971SSASJ..35..286K.
- ↑ Bürgmann H.; Widmer F.; Sigler W. V; Zeyer J. (2003). "mRNA Extraction and Reverse Transcription-PCR Protocol for Detection of nifH Gene Expression by Azotobacter vinelandii in Soil". Applied and Environmental Microbiology 69 (4): 1928–1935. doi:10.1128/AEM.69.4.1928-1935.2003. PMID 12676666. Bibcode: 2003ApEnM..69.1928B.
- ↑ Shank Yu; Demin O.; Bogachev AV (2005). "Respiratory Protection nitrogenase complex in Azotobacter vinelandii". Success Biological Chemistry 45: 205–234. http://www.inbi.ras.ru/ubkh/45/bertsova.pdf. Retrieved 2010-08-30.
- ↑ Maier R. J.; Moshiri F. (2000). "Role of the Azotobacter vinelandii Nitrogenase-Protective Shethna Protein in Preventing Oxygen-Mediated Cell Death". Journal of Bacteriology 182 (13): 3854–3857. doi:10.1128/JB.182.13.3854-3857.2000. PMID 10851006.
- ↑ Durrant M. C.; Francis A.; Lowe D. J.; Newton W. E.; Fisher K. (2006). "Evidence for a dynamic role for homocitrate during nitrogen fixation: the effect of substitution at the α-Lys426 position in MoFe-protein of Azotobacter vinelandii". Biochemical Journal 397 (2): 261–270. doi:10.1042/BJ20060102. PMID 16566750.
- ↑ Howard J. B.; Rees D. C. (2006). "How many metals does it take to fix N2? A mechanistic overview of biological nitrogen fixation". Proceedings of the National Academy of Sciences of the United States of America 103 (46): 17088–17093. doi:10.1073/pnas.0603978103. PMID 17088547. PMC 1859894. Bibcode: 2006PNAS..10317088H. http://authors.library.caltech.edu/9607/1/HOWpnas06.pdf. Retrieved 2018-11-04.
- ↑ Bellenger J. P.; Wichard T.; Kraepiel A. M. L. (2008). "Vanadium Requirements and Uptake Kinetics in the Dinitrogen-Fixing Bacterium Azotobacter vinelandii". Applied and Environmental Microbiology 74 (5): 1478–1484. doi:10.1128/AEM.02236-07. PMID 18192412. Bibcode: 2008ApEnM..74.1478B.
- ↑ Rüttimann-Johnson C.; Rubio L. M.; Dean D. R.; Ludden P. W. (2003). "VnfY Is Required for Full Activity of the Vanadium-Containing Dinitrogenase in Azotobacter vinelandii". Journal of Bacteriology 185 (7): 2383–2386. doi:10.1128/JB.185.7.2383-2386.2003. PMID 12644512.
- ↑ Robson R. L.; Eady R. R.; Richardson T. H.; Miller R. W.; Hawkins M.; Postgate J. R. (1986). "The alternative nitrogenase of Azotobacter chroococcum is a vanadium enzyme". Nature 322 (6077): 388–390. doi:10.1038/322388a0. Bibcode: 1986Natur.322..388R.
- ↑ Miller R. W.; Eady R. R. (1988). "Molybdenum and vanadium nitrogenases of Azotobacter chroococcum. Low temperature favours N2 reduction by vanadium nitrogenase". Biochemical Journal 256 (2): 429–432. doi:10.1042/bj2560429. PMID 3223922.
- ↑ Hu Y.; Fay A. W.; Lee C. C.; Ribbe M. W. (2007). "P-cluster maturation on nitrogenase MoFe protein". Proceedings of the National Academy of Sciences of the United States of America 104 (25): 10424–10429. doi:10.1073/pnas.0704297104. PMID 17563349. Bibcode: 2007PNAS..10410424H.
- ↑ Curatti L.; Brown C. S.; Ludden P. W.; Rubio L. M. (2005). "Genes required for rapid expression of nitrogenase activity in Azotobacter vinelandii". Proceedings of the National Academy of Sciences of the United States of America 102 (18): 6291–6296. doi:10.1073/pnas.0501216102. PMID 15845763. Bibcode: 2005PNAS..102.6291C.
- ↑ Hill S.; Austin S.; Eydmann T.; Jones T.; Dixon R. (1996). "Azotobacter vinelandii NIFL is a flavoprotein that modulates transcriptional activation of nitrogen-fixation genes via a redox-sensitive switch". Proceedings of the National Academy of Sciences of the United States of America 93 (5): 2143–2148. doi:10.1073/pnas.93.5.2143. PMID 8700899. Bibcode: 1996PNAS...93.2143H.
- ↑ Money T.; Barrett J.; Dixon R.; Austin S. (2001). "Protein-Protein Interactions in the Complex between the Enhancer Binding Protein NIFA and the Sensor NIFL from Azotobacter vinelandii". Journal of Bacteriology 183 (4): 1359–1368. doi:10.1128/JB.183.4.1359-1368.2001. PMID 11157949.
- ↑ Ahmad F.; Ahmad I.; Khan M. S. (2005). "Indole Acetic Acid Production by the Indigenous Isolates of Azotobacter and Fluorescent Pseudomonas in the Presence and Absence of Tryptophan". Turkish Journal of Biology (29): 29–34. http://journals.tubitak.gov.tr/biology/issues/biy-05-29-1/biy-29-1-5-0410-1.pdf.
- ↑ Oblisami G.; Santhanakrishan P.; Pappiah C. M.; Shabnugavelu K. G. (1985). "Effect of Azotobacter Inoculant And Growth Regulators on the Growth of Cashew". Acta Horticulturae (108): 44–49. doi:10.17660/actahortic.1985.108.7. http://www.actahort.org/books/108/108_7.htm. Retrieved 2010-08-30.
- ↑ Rajaee S.; Alikhani H. A.; Raiesi F. (2007). "Effect of Plant Growth Promoting Potentials of Azotobacter chroococcum Native Strains on Growth, Yield and Uptake of Nutrients in Wheat". Journal of Science and Technology of Agriculture and Natural Resources 11 (41): 297. http://jstnar.iut.ac.ir/browse.php?a_code=A-10-2-760&slc_lang=en&sid=1. Retrieved 2013-03-22. PDF copy
- ↑ Chen J. H.; Czajka D. R.; Lion L. W.; Shuler M. L.; Ghiorse W. C. (1995). "Trace metal mobilization in soil by bacterial polymers". Environmental Health Perspectives 103 (1): 53–58. doi:10.2307/3432013. PMID 7621800.
- ↑ Li D. Y.; Eberspächer J.; Wagner B.; Kuntzer J.; Lingens F. (1991). "Degradation of 2,4,6-trichlorophenol by Azotobacter sp. strain GP1". Applied and Environmental Microbiology 57 (7): 1920–1928. doi:10.1128/AEM.57.7.1920-1928.1991. PMID 1892382. Bibcode: 1991ApEnM..57.1920L.
- ↑ Neeru Narula, ed (2000). Azotobacter in Sustainable Agriculture. New Delhi. ISBN 978-81-239-0661-4. http://vedamsbooks.com/no15326.htm. Retrieved 2010-08-30.
- ↑ Galindo E.; Peña C.; Núñez C.; Segura D.; Espín G. (2007). "Molecular and bioengineering strategies to improve alginate and polydydroxyalkanoate production by Azotobacter vinelandii". Microbial Cell Factories 6 (7): 7. doi:10.1186/1475-2859-6-7. PMID 17306024.
- ↑ Page W. J.; Tindale A.; Chandra M.; Kwon E. (2001). "Alginate formation in Azotobacter vinelandii UWD during stationary phase and the turnover of poly-β-hydroxybutyrate". Microbiology 147 (Pt 2): 483–490. doi:10.1099/00221287-147-2-483. PMID 11158365.
- ↑ Ahmed M.; Ahmed N. (2007). "Genetics of Bacterial Alginate: Alginate Genes Distribution, Organization and Biosynthesis in Bacteria". Current Genomics 8 (3): 191–202. doi:10.2174/138920207780833810. PMID 18645604.
- ↑ Schlegel, Hans Günter; Zaborosch, C.; Kogut, M. (1993). General microbiology. Cambridge University Press. p. 380. ISBN 978-0-521-43980-0. https://books.google.com/books?id=DrHQtIbiunkC&pg=PA380.
- ↑ Beijerinck M. W. (1901). "Ueber Oligonitrophile Mikroben" (in de). Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene, Abteilung II (7): 561–582.
- ↑ Page W. J.; Shivprasad S. (1991). "Azotobacter salinestris sp. nov., a sodium-dependent, microaerophilic, and aeroadaptive nitrogen-fixing bacterium". International Journal of Systematic Bacteriology 41 (3): 369–376. doi:10.1099/00207713-41-3-369.
- ↑ Rediers H.; Vanderleyden J.; De Mot R. (2004). "Azotobacter vinelandii: a Pseudomonas in disguise?". Microbiology 150 (Pt 5): 1117–1119. doi:10.1099/mic.0.27096-0. PMID 15133068.
- ↑ Young J. M.; Park D.-C. (2007). "Probable synonymy of the nitrogen-fixing genus Azotobacter and the genus Pseudomonas". International Journal of Systematic and Evolutionary Microbiology 57 (Pt 12): 2894–2901. doi:10.1099/ijs.0.64969-0. PMID 18048745.
External links
- "Azotobacter". http://microbewiki.kenyon.edu/index.php/Azotobacter.
- Euzéby, J. P.. "Azotobacter Beijerinck 1901". List of Prokaryotic names with Standing in Nomenclature. https://lpsn.dsmz.de/genus/azotobacter.
- "Azotobacter.org" (A project to study the genome of Azotobacter vinelandii). http://www.azotobacter.org/.
- Crum, Amy. "Azotbacter". SOIL MICROBIOLOGY BIOL/CSES 4684. http://filebox.vt.edu/users/chagedor/biol_4684/Microbes/AZOTO.html.
- "Azotobacter vinelandii". John Innes Centre – Molecular Microbiology Department. http://www.jic.ac.uk/SCIENCE/molmicro/Azot.html.
- "Azotobacter vinelandii". JGI. http://genome.jgi-psf.org/draft_microbes/azovi/azovi.home.html.
- Iwao WATANABE (JICA/Cantho Univ. Expert Mar–Apr. 2000). (March 30, 2000). "Biological Nitrogen Fixation and its Use in Agriculture". Lecture in Cantho University, Vietnam. http://www.asahi-net.or.jp/~it6i-wtnb/BNF.html.
- "Azotobacter". Microbiology Video Library. MicrobiologyBytes. http://www.microbiologybytes.com/video/Azotobacter.html.
Wikidata ☰ Q132995 entry
 |
