Biology:Chromosome 15
| Chromosome 15 | |
|---|---|
 Human chromosome 15 pair after G-banding. One is from mother, one is from father. | |
 Chromosome 15 pair in human male karyogram. | |
| Features | |
| Length (bp) | 99,753,195 bp (CHM13) |
| No. of genes | 561 (CCDS)[1] |
| Type | Autosome |
| Centromere position | Acrocentric[2] (19.0 Mbp[3]) |
| Complete gene lists | |
| CCDS | Gene list |
| HGNC | Gene list |
| UniProt | Gene list |
| NCBI | Gene list |
| External map viewers | |
| Ensembl | Chromosome 15 |
| Entrez | Chromosome 15 |
| NCBI | Chromosome 15 |
| UCSC | Chromosome 15 |
| Full DNA sequences | |
| RefSeq | NC_000015 (FASTA) |
| GenBank | CM000677 (FASTA) |
Chromosome 15 is one of the 23 pairs of chromosomes in humans. People normally have two copies of this chromosome. Chromosome 15 spans about 99.7 million base pairs (the building material of DNA) and represents between 3% and 3.5% of the total DNA in cells. Chromosome 15 is an acrocentric chromosome, with a very small short arm (the "p" arm, for "petite"), which contains few protein coding genes among its 19 million base pairs. It has a larger long arm (the "q" arm) that is gene rich, spanning about 83 million base pairs.
The human leukocyte antigen gene for β2-microglobulin is found on chromosome 15, as well as the FBN1 gene, coding for both fibrillin-1 (a protein critical to the proper functioning of connective tissue), and asprosin (a small protein produced from part of the transcribed FBN1 gene mRNA), which is involved in fat metabolism.
Genes
Number of genes
The following are some of the gene count estimates of human chromosome 15. Because researchers use different approaches to genome annotation their predictions of the number of genes on each chromosome varies (for technical details, see gene prediction). Among various projects, the collaborative consensus coding sequence project (CCDS) takes an extremely conservative strategy. So CCDS's gene number prediction represents a lower bound on the total number of human protein-coding genes.[4]
| Estimated by | Protein-coding genes | Non-coding RNA genes | Pseudogenes | Source | Release date |
|---|---|---|---|---|---|
| CCDS | 561 | — | — | [1] | 2016-09-08 |
| HGNC | 559 | 328 | 433 | [5] | 2017-05-12 |
| Ensembl | 605 | 992 | 508 | [6] | 2017-03-29 |
| UniProt | 601 | — | — | [7] | 2018-02-28 |
| NCBI | 629 | 716 | 594 | [8][9][10] | 2017-05-19 |
Gene list
The following is a partial list of genes on human chromosome 15. For complete list, see the link in the infobox on the right.
Chromosomal conditions
The following conditions are caused by mutations in chromosome 15. Two of the conditions (Angelman syndrome and Prader–Willi syndrome) involve a loss of gene activity in the same part of chromosome 15, the 15q11.2-q13.1 region. This discovery provided the first evidence in humans that something beyond genes could determine how the genes are expressed.[11]
Angelman syndrome
The main characteristics of Angelman syndrome are severe intellectual disability, ataxia, lack of speech, and excessively happy demeanor. Angelman syndrome results from a loss of gene activity in a specific part of chromosome 15, the 15q11-q13 region. This region contains a gene called UBE3A that, when mutated or absent, likely causes the characteristic features of this condition. People normally have two copies of the UBE3A gene, one from each parent. Both copies of this gene are active in many of the body's tissues. In the brain, however, only the copy inherited from a person's mother (the maternal copy) is active. If the maternal copy is lost because of a chromosomal change or a gene mutation, a person will have no working copies of the UBE3A gene in the brain.
In most cases (about 70%)[citation needed], people with Angelman syndrome have a deletion in the maternal copy of chromosome 15. This chromosomal change deletes the region of chromosome 15 that includes the UBE3A gene. Because the copy of the UBE3A gene inherited from a person's father (the paternal copy) is normally inactive in the brain, a deletion in the maternal chromosome 15 results in no active copies of the UBE3A gene in the brain.
In 3% to 7% of cases,[citation needed] Angelman syndrome occurs when a person has two copies of the paternal chromosome 15 instead of one copy from each parent. This phenomenon is called paternal uniparental disomy (UPD). People with paternal UPD for chromosome 15 have two copies of the UBE3A gene, but they are both inherited from the father and are therefore inactive in the brain.
About 10% of Angelman syndrome cases are caused by a mutation in the UBE3A gene, and another 3% result from a defect in the DNA region that controls the activation of the UBE3A gene and other genes on the maternal copy of chromosome 15. In a small percentage of cases, Angelman syndrome may be caused by a chromosomal rearrangement called a translocation or by a mutation in a gene other than UBE3A. These genetic changes can abnormally inactivate the UBE3A gene.
Angelman syndrome can be hereditary, as evidenced by one case where a patient became pregnant with a daughter who also had the condition.[12]
Prader–Willi syndrome
The main characteristics of this condition include polyphagia (extreme, insatiable appetite), mild to moderate developmental delay, hypogonadism resulting in delayed to no puberty, and hypotonia. Prader-Willi syndrome is caused by the loss of active genes in a specific part of chromosome 15, the 15q11-q13 region. People normally have two copies of this chromosome in each cell, one copy from each parent. Prader–Willi syndrome occurs when the paternal copy is partly or entirely missing.
In about 70% of cases,[citation needed] Prader–Willi syndrome occurs when the 15q11-q13 region of the paternal chromosome 15 is deleted. The genes in this region are normally active on the paternal copy of the chromosome and are inactive on the maternal copy. Therefore, a person with a deletion in the paternal chromosome 15 will have no active genes in this region.
In about 25% of cases, a person with Prader–Willi syndrome has two maternal copies of chromosome 15 in each cell instead of one copy from each parent. This phenomenon is called maternal uniparental disomy. Because some genes are normally active only on the paternal copy of this chromosome, a person with two maternal copies of chromosome 15 will have no active copies of these genes.
In a small percentage of cases, Prader–Willi syndrome is not caused by a chromosomal rearrangement called a translocation. Rarely, the condition is caused by an abnormality in the DNA region that controls the activity of genes on the paternal chromosome 15. Because patients almost always have difficulty reproducing, Prader–Willi syndrome is generally not hereditary.
Isodicentric chromosome 15
A specific chromosomal change called an isodicentric chromosome 15 (IDIC15) (also known by a number of other names) can affect growth and development. The patient possesses an "extra" or "marker" chromosome. This small extra chromosome is made up of genetic material from chromosome 15 that has been abnormally duplicated (copied) and attached end-to-end. In some cases, the extra chromosome is very small and has no effect on a person's health. A larger isodicentric chromosome 15 can result in weak muscle tone (hypotonia), intellectual disability, seizures, and behavioral problems.[13] Signs and symptoms of autism (a developmental disorder that affects communication and social interaction) have also been associated with the presence of an isodicentric chromosome 15.
Other chromosomal conditions
Other changes in the number or structure of chromosome 15 can cause developmental delays, delayed growth and development, hypotonia, and characteristic facial features.[citation needed] These changes include an extra copy of part of chromosome 15 in each cell (partial trisomy 15) or a missing segment of the chromosome in each cell (partial monosomy 15). In some cases, several of the chromosome's DNA building blocks (nucleotides) are deleted or duplicated.
The following diseases are some of those related to genes on chromosome 15:[citation needed]
- Bloom syndrome
- Breast cancer
- Isovaleric acidemia
- Loeys–Dietz, type 3 (SMAD3 gene)
- Marfan syndrome
- Nonsyndromic deafness
- Schaaf–Yang syndrome (SYS)
- Tay–Sachs disease
- Tyrosinemia
- Autosomal Dominant Compelling Helio-Ophthalmic Outburst syndrome[14]
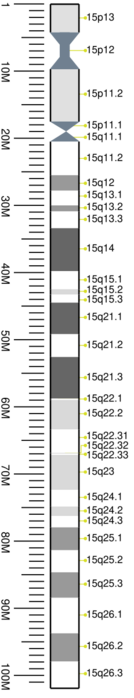
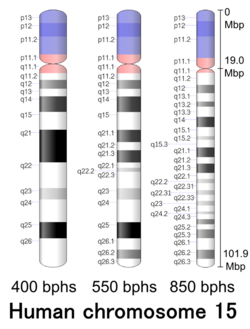
Cytogenetic band
| Chr. | Arm[19] | Band[20] | ISCN start[21] |
ISCN stop[21] |
Basepair start |
Basepair stop |
Stain[22] | Density |
|---|---|---|---|---|---|---|---|---|
| 15 | p | 13 | 0 | 270 | 1 | 4,200,000 | gvar | |
| 15 | p | 12 | 270 | 631 | 4,200,001 | 9,700,000 | stalk | |
| 15 | p | 11.2 | 631 | 1142 | 9,700,001 | 17,500,000 | gvar | |
| 15 | p | 11.1 | 1142 | 1382 | 17,500,001 | 19,000,000 | acen | |
| 15 | q | 11.1 | 1382 | 1487 | 19,000,001 | 20,500,000 | acen | |
| 15 | q | 11.2 | 1487 | 1773 | 20,500,001 | 25,500,000 | gneg | |
| 15 | q | 12 | 1773 | 1968 | 25,500,001 | 27,800,000 | gpos | 50 |
| 15 | q | 13.1 | 1968 | 2164 | 27,800,001 | 30,000,000 | gneg | |
| 15 | q | 13.2 | 2164 | 2284 | 30,000,001 | 30,900,000 | gpos | 50 |
| 15 | q | 13.3 | 2284 | 2524 | 30,900,001 | 33,400,000 | gneg | |
| 15 | q | 14 | 2524 | 2765 | 33,400,001 | 39,800,000 | gpos | 75 |
| 15 | q | 15.1 | 2765 | 2975 | 39,800,001 | 42,500,000 | gneg | |
| 15 | q | 15.2 | 2975 | 3065 | 42,500,001 | 43,300,000 | gpos | 25 |
| 15 | q | 15.3 | 3065 | 3245 | 43,300,001 | 44,500,000 | gneg | |
| 15 | q | 21.1 | 3245 | 3471 | 44,500,001 | 49,200,000 | gpos | 75 |
| 15 | q | 21.2 | 3471 | 3621 | 49,200,001 | 52,600,000 | gneg | |
| 15 | q | 21.3 | 3621 | 3846 | 52,600,001 | 58,800,000 | gpos | 75 |
| 15 | q | 22.1 | 3846 | 3982 | 58,800,001 | 59,000,000 | gneg | |
| 15 | q | 22.2 | 3982 | 4087 | 59,000,001 | 63,400,000 | gpos | 25 |
| 15 | q | 22.31 | 4087 | 4252 | 63,400,001 | 66,900,000 | gneg | |
| 15 | q | 22.32 | 4252 | 4357 | 66,900,001 | 67,000,000 | gpos | 25 |
| 15 | q | 22.33 | 4357 | 4507 | 67,000,001 | 67,200,000 | gneg | |
| 15 | q | 23 | 4507 | 4613 | 67,200,001 | 72,400,000 | gpos | 25 |
| 15 | q | 24.1 | 4613 | 4748 | 72,400,001 | 74,900,000 | gneg | |
| 15 | q | 24.2 | 4748 | 4808 | 74,900,001 | 76,300,000 | gpos | 25 |
| 15 | q | 24.3 | 4808 | 4928 | 76,300,001 | 78,000,000 | gneg | |
| 15 | q | 25.1 | 4928 | 5048 | 78,000,001 | 81,400,000 | gpos | 50 |
| 15 | q | 25.2 | 5048 | 5169 | 81,400,001 | 84,700,000 | gneg | |
| 15 | q | 25.3 | 5169 | 5379 | 84,700,001 | 88,500,000 | gpos | 50 |
| 15 | q | 26.1 | 5379 | 5649 | 88,500,001 | 93,800,000 | gneg | |
| 15 | q | 26.2 | 5649 | 5860 | 93,800,001 | 98,000,000 | gpos | 50 |
| 15 | q | 26.3 | 5860 | 6070 | 98,000,001 | 101,991,189 | gneg |
References
Specific references:
- ↑ 1.0 1.1 "Search results – 15[CHR] AND "Homo sapiens"[Organism] AND ("has ccds"[Properties] AND alive[prop]) – Gene". 2016-09-08. https://www.ncbi.nlm.nih.gov/gene?term=15%5BChr%5D%20AND%20%22Homo%20sapiens%22%5BOrganism%5D%20AND%20%28%22has%20ccds%22%5BProperties%5D%20AND%20alive%5Bprop%5D%29&cmd=DetailsSearch.
- ↑ Tom Strachan; Andrew Read (2 April 2010). Human Molecular Genetics. Garland Science. p. 45. ISBN 978-1-136-84407-2. https://books.google.com/books?id=dSwWBAAAQBAJ&pg=PA45.
- ↑ 3.0 3.1 3.2 Genome Decoration Page, NCBI. Ideogram data for Homo sapience (850 bphs, Assembly GRCh38.p3). Last update 2014-06-03. Retrieved 2017-04-26.
- ↑ Pertea M, Salzberg SL (2010). "Between a chicken and a grape: estimating the number of human genes.". Genome Biol 11 (5): 206. doi:10.1186/gb-2010-11-5-206. PMID 20441615.
- ↑ "Statistics & Downloads for chromosome 15". 2017-05-12. https://www.genenames.org/cgi-bin/statistics?c=15.
- ↑ "Chromosome 15: Chromosome summary – Homo sapiens". 2017-03-29. http://mar2017.archive.ensembl.org/Homo_sapiens/Location/Chromosome?r=15.
- ↑ "Human chromosome 15: entries, gene names and cross-references to MIM". 2018-02-28. https://www.uniprot.org/docs/humchr15.txt.
- ↑ "Search results – 15[CHR] AND "Homo sapiens"[Organism] AND ("genetype protein coding"[Properties] AND alive[prop]) – Gene". 2017-05-19. https://www.ncbi.nlm.nih.gov/gene?term=15%5BCHR%5D%20AND%20%22Homo%20sapiens%22%5BOrganism%5D%20AND%20%28%22genetype%20protein%20coding%22%5BProperties%5D%20AND%20alive%5Bprop%5D%29&cmd=DetailsSearch.
- ↑ "Search results – 15[CHR] AND "Homo sapiens"[Organism] AND ( ("genetype miscrna"[Properties] OR "genetype ncrna"[Properties] OR "genetype rrna"[Properties] OR "genetype trna"[Properties] OR "genetype scrna"[Properties] OR "genetype snrna"[Properties] OR "genetype snorna"[Properties]) NOT "genetype protein coding"[Properties] AND alive[prop]) – Gene". 2017-05-19. https://www.ncbi.nlm.nih.gov/gene?term=15%5BCHR%5D%20AND%20%22Homo%20sapiens%22%5BOrganism%5D%20AND%20%28%28%22genetype%20miscrna%22%5BProperties%5D%20OR%20%22genetype%20ncrna%22%5BProperties%5D%20OR%20%22genetype%20rrna%22%5BProperties%5D%20OR%20%22genetype%20trna%22%5BProperties%5D%20OR%20%22genetype%20scrna%22%5BProperties%5D%20OR%20%22genetype%20snrna%22%5BProperties%5D%20OR%20%22genetype%20snorna%22%5BProperties%5D%29%20NOT%20%22genetype%20protein%20coding%22%5BProperties%5D%20AND%20alive%5Bprop%5D%29&cmd=DetailsSearch.
- ↑ "Search results – 15[CHR] AND "Homo sapiens"[Organism] AND ("genetype pseudo"[Properties] AND alive[prop]) – Gene". 2017-05-19. https://www.ncbi.nlm.nih.gov/gene?term=15%5BCHR%5D%20AND%20%22Homo%20sapiens%22%5BOrganism%5D%20AND%20%28%22genetype%20pseudo%22%5BProperties%5D%20AND%20alive%5Bprop%5D%29&cmd=DetailsSearch.
- ↑ "Teacher's Guide". Ghost in Your Genes (season 35). Nova (TV series). October 16, 2007. https://www.pbs.org/wgbh/nova/teachers/programs/3413_genes.html. "The program...recounts how one scientist determined how the deletion of a key sequence of DNA on human chromosome 15 could lead to two different syndromes depending on whether the deletion originated from the mother or the father [and] explains that this was the first human evidence that something other than genes themselves could determine how genes are expressed."
- ↑ "Transmission of Angelman syndrome by an affected mother". Genet Med 1 (6): 262–6. 1999. doi:10.1097/00125817-199909000-00004. PMID 11258627.
- ↑ "What is Dup15q Syndrome? – Dup15q" (in en). http://www.dup15q.org/understanding-dup15q/what-is-dup15q-syndrome/.
- ↑ "Photic Sneeze Reflex | AncestryDNA® Traits Learning Hub" (in en). https://www.ancestry.com/lp/traits/photic-sneeze-reflex.
- ↑ Genome Decoration Page, NCBI. Ideogram data for Homo sapience (400 bphs, Assembly GRCh38.p3). Last update 2014-03-04. Retrieved 2017-04-26.
- ↑ Genome Decoration Page, NCBI. Ideogram data for Homo sapience (550 bphs, Assembly GRCh38.p3). Last update 2015-08-11. Retrieved 2017-04-26.
- ↑ International Standing Committee on Human Cytogenetic Nomenclature (2013). ISCN 2013: An International System for Human Cytogenetic Nomenclature (2013). Karger Medical and Scientific Publishers. ISBN 978-3-318-02253-7. https://books.google.com/books?id=lGCLrh0DIwEC.
- ↑ Sethakulvichai, W.; Manitpornsut, S.; Wiboonrat, M.; Lilakiatsakun, W.; Assawamakin, A.; Tongsima, S. (2012). "Estimation of band level resolutions of human chromosome images". 2012 Ninth International Conference on Computer Science and Software Engineering (JCSSE). pp. 276–282. doi:10.1109/JCSSE.2012.6261965. ISBN 978-1-4673-1921-8. https://www.researchgate.net/publication/261304470.
- ↑ "p": Short arm; "q": Long arm.
- ↑ For cytogenetic banding nomenclature, see article locus.
- ↑ 21.0 21.1 These values (ISCN start/stop) are based on the length of bands/ideograms from the ISCN book, An International System for Human Cytogenetic Nomenclature (2013). Arbitrary unit.
- ↑ gpos: Region which is positively stained by G banding, generally AT-rich and gene poor; gneg: Region which is negatively stained by G banding, generally CG-rich and gene rich; acen Centromere. var: Variable region; stalk: Stalk.
General references:
- "Prader-Willi syndrome: clinical genetics, cytogenetics and molecular biology". Expert Rev Mol Med 7 (14): 1–20. 2005. doi:10.1017/S1462399405009531. PMID 16038620.
- "Microarray analysis of gene/transcript expression in Prader-Willi syndrome: deletion versus UPD". J Med Genet 40 (8): 568–574. 2003. doi:10.1136/jmg.40.8.568. PMID 12920063.
- "Microarray analysis of gene/transcript expression in Angelman syndrome: deletion versus UPD". Genomics 85 (1): 85–91. 2005. doi:10.1016/j.ygeno.2004.10.010. PMID 15607424.
- "Relationship between clinical and genetic features in "inverted duplicated chromosome 15" patients". Pediatr Neurol 24 (2): 111–116. 2001. doi:10.1016/S0887-8994(00)00244-7. PMID 11275459.
- "Behavioral differences among subjects with Prader-Willi syndrome and type I or type II deletion and maternal disomy". Pediatrics 113 (3 Pt 1): 565–573. 2004. doi:10.1542/peds.113.3.565. PMID 14993551.
- "Prader-Willi and Angelman syndromes: sister imprinted disorders". Am J Med Genet 97 (2): 136–146. 2000. doi:10.1002/1096-8628(200022)97:2<136::AID-AJMG5>3.0.CO;2-V. PMID 11180221.
- "Angelman syndrome: a review of the clinical and genetic aspects". J Med Genet 40 (2): 87–95. 2003. doi:10.1136/jmg.40.2.87. PMID 12566516.
- Gilbert F (1999). "Disease genes and chromosomes: disease maps of the human genome. Chromosome 15". Genet Test 3 (3): 309–322. doi:10.1089/109065799316653. PMID 10495933.
- "Identification of novel imprinted transcripts in the Prader-Willi syndrome and Angelman syndrome deletion region: further evidence for regional imprinting control". Am J Hum Genet 66 (3): 848–858. 2000. doi:10.1086/302817. PMID 10712201.
- "Autistic symptoms among children and young adults with isodicentric chromosome 15". Am J Med Genet 81 (5): 428–433. 1998. doi:10.1002/(SICI)1096-8628(19980907)81:5<428::AID-AJMG12>3.0.CO;2-E. PMID 9754629.
- "Partial duplication of the long arm of chromosome 15: confirmation of a causative role in craniosynostosis and definition of a 15q25-qter trisomy syndrome". Am J Med Genet 87 (5): 391–394. 1999. doi:10.1002/(SICI)1096-8628(19991222)87:5<391::AID-AJMG4>3.0.CO;2-O. PMID 10594876.
External links
- National Institutes of Health. "Chromosome 15". Genetics Home Reference. http://ghr.nlm.nih.gov/chromosome=15.
- "Chromosome 15". http://web.ornl.gov/sci/techresources/Human_Genome/posters/chromosome/chromo15.shtml.
 |