Biology:Crassulacean acid metabolism

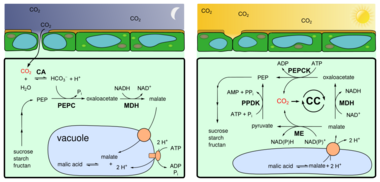
Crassulacean acid metabolism, also known as CAM photosynthesis, is a carbon fixation pathway that evolved in some plants as an adaptation to arid conditions[1] that allows a plant to photosynthesize during the day, but only exchange gases at night. In a plant using full CAM, the stomata in the leaves remain shut during the day to reduce evapotranspiration, but they open at night to collect carbon dioxide (CO
2) and allow it to diffuse into the mesophyll cells. The CO
2 is stored as four-carbon malic acid in vacuoles at night, and then in the daytime, the malate is transported to chloroplasts where it is converted back to CO
2, which is then used during photosynthesis. The pre-collected CO
2 is concentrated around the enzyme RuBisCO, increasing photosynthetic efficiency. This mechanism of acid metabolism was first discovered in plants of the family Crassulaceae.
Historical background
Observations relating to CAM were first made by de Saussure in 1804 in his Recherches Chimiques sur la Végétation.[2] Benjamin Heyne in 1812 noted that Bryophyllum leaves in India were acidic in the morning and tasteless by afternoon.[3] These observations were studied further and refined by Aubert, E. in 1892 in his Recherches physiologiques sur les plantes grasses and expounded upon by Richards, H. M. 1915 in Acidity and Gas Interchange in Cacti, Carnegie Institution. The term CAM may have been coined by Ranson and Thomas in 1940, but they were not the first to discover this cycle. It was observed by the botanists Ranson and Thomas, in the succulent family Crassulaceae (which includes jade plants and Sedum).[4] The name "Crassulacean acid metabolism" refers to acid metabolism in Crassulaceae, and not the metabolism of "crassulacean acid"; there is no chemical by that name.
Overview: a two-part cycle
CAM is an adaptation for increased efficiency in the use of water, and so is typically found in plants growing in arid conditions.[5] (CAM is found in over 99% of the known 1700 species of Cactaceae and in nearly all of the cacti producing edible fruits.)[6]
During the night
During the night, a plant employing CAM has its stomata open, allowing CO
2 to enter and be fixed as organic acids by a PEP reaction similar to the C4 pathway. The resulting organic acids are stored in vacuoles for later use, as the Calvin cycle cannot operate without ATP and NADPH, products of light-dependent reactions that do not take place at night.[7]
During the day
During the day, the stomata close to conserve water, and the CO
2-storing organic acids are released from the vacuoles of the mesophyll cells. An enzyme in the stroma of chloroplasts releases the CO
2, which enters into the Calvin cycle so that photosynthesis may take place.[citation needed]
Benefits
The most important benefit of CAM to the plant is the ability to leave most leaf stomata closed during the day.[8] Plants employing CAM are most common in arid environments, where water is scarce. Being able to keep stomata closed during the hottest and driest part of the day reduces the loss of water through evapotranspiration, allowing such plants to grow in environments that would otherwise be far too dry. Plants using only C3 carbon fixation, for example, lose 97% of the water they take up through the roots to transpiration - a high cost avoided by plants able to employ CAM.[9][What percentage is lost in CAM plants?]
Comparison with C4 metabolism
This section does not cite any external source. HandWiki requires at least one external source. See citing external sources. (October 2019) (Learn how and when to remove this template message) |
The C4 pathway bears resemblance to CAM; both act to concentrate CO
2 around RuBisCO, thereby increasing its efficiency. CAM concentrates it temporally, providing CO
2 during the day, and not at night, when respiration is the dominant reaction. C4 plants, in contrast, concentrate CO
2 spatially, with a RuBisCO reaction centre in a "bundle sheath cell" being inundated with CO
2. Due to the inactivity required by the CAM mechanism, C4 carbon fixation has a greater efficiency in terms of PGA synthesis.
There are some C4/CAM intermediate species, such as Peperomia camptotricha, Portulaca oleracea, and Portulaca grandiflora. It was previously thought that the two pathways of photosynthesis in such plants could occur in the same leaves but not in the same cells, and that the two pathways could not couple but only occur side by side.[10] It is now known, however, that in at least some species such as Portulaca oleracea, C4 and CAM photosynthesis are fully integrated within the same cells, and that CAM-generated metabolites are incorporated directly into the C4 cycle.[11]
Biochemistry
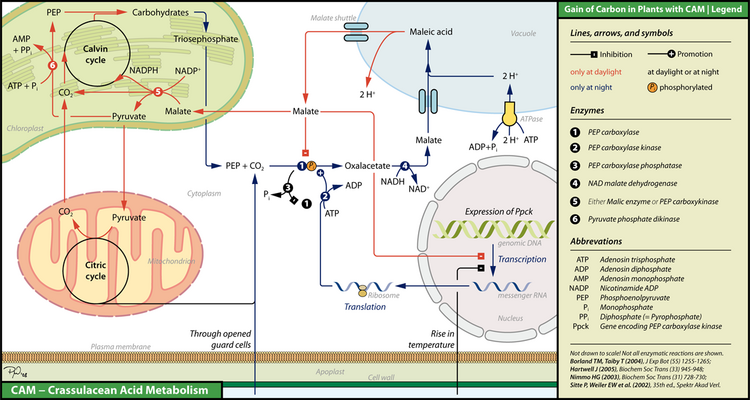
Plants with CAM must control storage of CO
2 and its reduction to branched carbohydrates in space and time.
At low temperatures (frequently at night), plants using CAM open their stomata, CO
2 molecules diffuse into the spongy mesophyll's intracellular spaces and then into the cytoplasm. Here, they can meet phosphoenolpyruvate (PEP), which is a phosphorylated triose. During this time, the plants are synthesizing a protein called PEP carboxylase kinase (PEP-C kinase), whose expression can be inhibited by high temperatures (frequently at daylight) and the presence of malate. PEP-C kinase phosphorylates its target enzyme PEP carboxylase (PEP-C). Phosphorylation dramatically enhances the enzyme's capability to catalyze the formation of oxaloacetate, which can be subsequently transformed into malate by NAD+ malate dehydrogenase. Malate is then transported via malate shuttles into the vacuole, where it is converted into the storage form malic acid. In contrast to PEP-C kinase, PEP-C is synthesized all the time but almost inhibited at daylight either by dephosphorylation via PEP-C phosphatase or directly by binding malate. The latter is not possible at low temperatures, since malate is efficiently transported into the vacuole, whereas PEP-C kinase readily inverts dephosphorylation.
In daylight, plants using CAM close their guard cells and discharge malate that is subsequently transported into chloroplasts. There, depending on plant species, it is cleaved into pyruvate and CO
2 either by malic enzyme or by PEP carboxykinase. CO
2 is then introduced into the Calvin cycle, a coupled and self-recovering enzyme system, which is used to build branched carbohydrates. The by-product pyruvate can be further degraded in the mitochondrial citric acid cycle, thereby providing additional CO
2 molecules for the Calvin Cycle. Pyruvate can also be used to recover PEP via pyruvate phosphate dikinase, a high-energy step, which requires ATP and an additional phosphate. During the following cool night, PEP is finally exported into the cytoplasm, where it is involved in fixing carbon dioxide via malate.
Use by plants
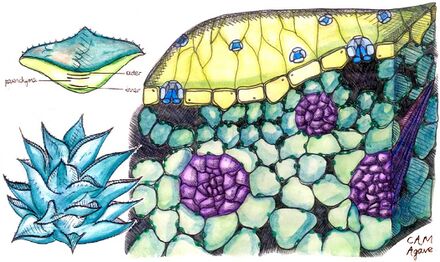
Plants use CAM to different degrees. Some are "obligate CAM plants", i.e. they use only CAM in photosynthesis, although they vary in the amount of CO
2 they are able to store as organic acids; they are sometimes divided into "strong CAM" and "weak CAM" plants on this basis. Other plants show "inducible CAM", in which they are able to switch between using either the C3 or C4 mechanism and CAM depending on environmental conditions. Another group of plants employ "CAM-cycling", in which their stomata do not open at night; the plants instead recycle CO
2 produced by respiration as well as storing some CO
2 during the day.[5]
Plants showing inducible CAM and CAM-cycling are typically found in conditions where periods of water shortage alternate with periods when water is freely available. Periodic drought – a feature of semi-arid regions – is one cause of water shortage. Plants which grow on trees or rocks (as epiphytes or lithophytes) also experience variations in water availability. Salinity, high light levels and nutrient availability are other factors which have been shown to induce CAM.[5]
Since CAM is an adaptation to arid conditions, plants using CAM often display other xerophytic characters, such as thick, reduced leaves with a low surface-area-to-volume ratio; thick cuticle; and stomata sunken into pits. Some shed their leaves during the dry season; others (the succulents[12]) store water in vacuoles. CAM also causes taste differences: plants may have an increasingly sour taste during the night yet become sweeter-tasting during the day. This is due to malic acid being stored in the vacuoles of the plants' cells during the night and then being used up during the day.[13]
Aquatic CAM
CAM photosynthesis is also found in aquatic species in at least 4 genera, including: Isoetes, Crassula, Littorella, Sagittaria, and possibly Vallisneria,[14] being found in a variety of species e.g. Isoetes howellii, Crassula aquatica.
These plants follow the same nocturnal acid accumulation and daytime deacidification as terrestrial CAM species.[15] However, the reason for CAM in aquatic plants is not due to a lack of available water, but a limited supply of CO
2.[14] CO
2 is limited due to slow diffusion in water, 10000x slower than in air. The problem is especially acute under acid pH, where the only inorganic carbon species present is CO
2, with no available bicarbonate or carbonate supply.
Aquatic CAM plants capture carbon at night when it is abundant due to a lack of competition from other photosynthetic organisms.[15] This also results in lowered photorespiration due to less photosynthetically generated oxygen.
Aquatic CAM is most marked in the summer months when there is increased competition for CO
2, compared to the winter months. However, in the winter months CAM still has a significant role.[16]
Ecological and taxonomic distribution of CAM-using plants
The majority of plants possessing CAM are either epiphytes (e.g., orchids, bromeliads) or succulent xerophytes (e.g., cacti, cactoid Euphorbias), but CAM is also found in hemiepiphytes (e.g., Clusia); lithophytes (e.g., Sedum, Sempervivum); terrestrial bromeliads; wetland plants (e.g., Isoetes, Crassula (Tillaea), Lobelia);[17] and in one halophyte, Mesembryanthemum crystallinum; one non-succulent terrestrial plant, (Dodonaea viscosa) and one mangrove associate (Sesuvium portulacastrum).
The only trees that can do CAM are in the genus Clusia; species of which are found across Central America, South America and the Caribbean. In Clusia, CAM is found in species that inhabit hotter, drier ecological niches, whereas species living in cooler montane forests tend to be C3.[18] In addition, some species of Clusia can temporarily switch their photosynthetic physiology from C3 to CAM, a process known as facultative CAM. This allows these trees to benefit from the elevated growth rates of C3 photosynthesis, when water is plentiful, and the drought tolerant nature of CAM, when the dry season occurs.
Plants which are able to switch between different methods of carbon fixation include Portulacaria afra, better known as Dwarf Jade Plant, which normally uses C3 fixation but can use CAM if it is drought-stressed,[19] and Portulaca oleracea, better known as Purslane, which normally uses C4 fixation but is also able to switch to CAM when drought-stressed.[20]
CAM has evolved convergently many times.[21] It occurs in 16,000 species (about 7% of plants), belonging to over 300 genera and around 40 families, but this is thought to be a considerable underestimate.[22] The great majority of plants using CAM are angiosperms (flowering plants) but it is found in ferns, Gnetopsida and in quillworts (relatives of club mosses). Interpretation of the first quillwort genome in 2021 (I. taiwanensis) suggested that its use of CAM was another example of convergent evolution.[23]
The following list summarizes the taxonomic distribution of plants with CAM:
| Division | Class/Angiosperm group | Order | Family | Plant Type | Clade involved |
|---|---|---|---|---|---|
| Lycopodiophyta | Isoetopsida | Isoetales | Isoetaceae | hydrophyte | Isoetes[24] (the sole genus of class Isoetopsida) - I. howellii (seasonally submerged), I. macrospora, I. bolanderi, I. engelmannii, I. lacustris, I. sinensis, I. storkii, I. kirkii, I. taiwanensis. |
| Pteridophyta | Polypodiopsida | Polypodiales | Polypodiaceae | epiphyte, lithophyte | CAM is recorded from Microsorum, Platycerium and Polypodium,[25] Pyrrosia and Drymoglossum[26] and Microgramma |
| Pteridopsida | Polypodiales | Pteridaceae[27] | epiphyte | Vittaria[28]
Anetium citrifolium[29] | |
| Cycadophyta | Cycadopsida | Cycadales | Zamiaceae | Dioon edule[30] | |
| Gnetophyta | Gnetopsida | Welwitschiales | Welwitschiaceae | xerophyte | Welwitschia mirabilis[31] (the sole species of the order Welwitschiales) |
| Magnoliophyta | magnoliids | Magnoliales | Piperaceae | epiphyte | Peperomia camptotricha[32] |
| eudicots | Caryophyllales | Aizoaceae | xerophyte | widespread in the family; Mesembryanthemum crystallinum is a rare instance of an halophyte that displays CAM[33] | |
| Cactaceae | xerophyte | Almost all cacti have obligate Crassulacean Acid Metabolism in their stems; the few cacti with leaves may have C3 Metabolism in those leaves;[34] seedlings have C3 Metabolism.[35] | |||
| Portulacaceae | xerophyte | recorded in approximately half of the genera (note: Portulacaceae is paraphyletic with respect to Cactaceae and Didiereaceae)[36] | |||
| Didiereaceae | xerophyte | ||||
| Saxifragales | Crassulaceae | hydrophyte, xerophyte, lithophyte | Crassulacean acid metabolism is widespread among the (eponymous) Crassulaceae. | ||
| eudicots (rosids) | Vitales | Vitaceae[37] | Cissus,[38] Cyphostemma | ||
| Malpighiales | Clusiaceae | hemiepiphyte | Clusia[38][39] | ||
| Euphorbiaceae[37] | CAM is found is some species of Euphorbia[38][40] including some formerly placed in the sunk genera Monadenium,[38] Pedilanthus[40] and Synadenium. C4 photosynthesis is also found in Euphorbia (subgenus Chamaesyce). | ||||
| Passifloraceae[27] | xerophyte | Adenia[41] | |||
| Geraniales | Geraniaceae | CAM is found in some succulent species of Pelargonium,[42] and is also reported from Geranium pratense[43] | |||
| Cucurbitales | Cucurbitaceae | Xerosicyos danguyi,[44] Dendrosicyos socotrana,[45] Momordica[46] | |||
| Celastrales | Celastraceae[47] | ||||
| Oxalidales | Oxalidaceae[48] | Oxalis carnosa var. hirta[48] | |||
| Brassicales | Moringaceae | Moringa[49] | |||
| Salvadoraceae[48] | CAM is found in Salvadora persica.[48] Salvadoraceae were previously placed in order Celastrales, but are now placed in Brassicales. | ||||
| Sapindales | Sapindaceae | Dodonaea viscosa | |||
| Fabales | Fabaceae[48] | CAM is found in Prosopis juliflora (listed under the family Salvadoraceae in Sayed's (2001) table,[48]) but is currently in the family Fabaceae (Leguminosae) according to The Plant List[50]). | |||
| Zygophyllaceae | Zygophyllum[49] | ||||
| eudicots (asterids) | Ericales | Ebenaceae | |||
| Solanales | Convolvulaceae | Ipomoea (Some species of Ipomoea are C3[38][51] - a citation is needed here.) | |||
| Gentianales | Rubiaceae | epiphyte | Hydnophytum and Myrmecodia | ||
| Apocynaceae | |||||
| Lamiales | Gesneriaceae | epiphyte | CAM was found Codonanthe crassifolia, but not in 3 other genera[52] | ||
| Lamiaceae | Plectranthus marrubioides, Coleus[citation needed] | ||||
| Plantaginaceae | hydrophyte | Littorella uniflora[24] | |||
| Apiales | Apiaceae | hydrophyte | Lilaeopsis lacustris | ||
| Asterales | Asteraceae[37] | some species of Senecio[53] | |||
| monocots | Alismatales | Hydrocharitaceae | hydrophyte | Hydrilla,[37] Vallisneria | |
| Alismataceae | hydrophyte | Sagittaria | |||
| Araceae | Zamioculcas zamiifolia is the only CAM plant in Araceae, and the only non-aquatic CAM plant in Alismatales[54] | ||||
| Poales | Bromeliaceae | epiphyte | Bromelioideae (91%), Puya (24%), Dyckia and related genera (all), Hechtia (all), Tillandsia (many)[55] | ||
| Cyperaceae | hydrophyte | Scirpus,[37] Eleocharis | |||
| Asparagales | Orchidaceae | epiphyte | Orchidaceae has more CAM species than any other family (CAM Orchids) | ||
| Agavaceae[39] | xerophyte | Agave,[38] Hesperaloe, Yucca and Polianthes[41] | |||
| Asphodelaceae[37] | xerophyte | Aloe,[38] Gasteria,[38] and Haworthia | |||
| Ruscaceae[37] | Sansevieria[38][48] (This genus is listed under the family Dracaenaceae in Sayed's (2001) table, but currently in the family Asparagaceae according to The Plant List), Dracaena[56] | ||||
| Commelinales | Commelinaceae | Callisia,[38] Tradescantia, Tripogandra |
See also
- C2 photosynthesis
- C3 carbon fixation
- C4 carbon fixation
- RuBisCO
References
- ↑ C. Michael Hogan. 2011. Respiration. Encyclopedia of Earth. Eds. Mark McGinley & C.J.cleveland. National council for Science and the Environment. Washington DC
- ↑ Recherches chimiques sur la végétation. Paris: Nyon. 1804.
- ↑ "The Role of Carbon Dioxide in Acid Formation by Succulent Plants". American Journal of Botany 35 (2): 113–117. 1948. doi:10.2307/2437894.
- ↑ "Crassulacean acid metabolism". Annual Review of Plant Physiology 11 (1): 81–110. 1960. doi:10.1146/annurev.pp.11.060160.000501. https://repository.arizona.edu/bitstream/10150/552219/1/dp_05_04-192.pdf.
- ↑ 5.0 5.1 5.2 "Crassulacean acid metabolism and fitness under water deficit stress: if not for carbon gain, what is facultative CAM good for?", Annals of Botany 103 (4): 645–653, 2008, doi:10.1093/aob/mcn145, PMID 18708641
- ↑ The Encyclopedia of Fruit & Nuts. CABI. 2008. p. 218.
- ↑ "The Ecology of Photosynthetic Pathways". Knowledge Project. Nature Education. 2010. https://www.nature.com/scitable/knowledge/library/the-ecology-of-photosynthetic-pathways-15785165/. "In this pathway, stomata open at night, which allows CO2 to diffuse into the leaf to be combined with PEP and form malate. This acid is then stored in large central vacuoles until daytime."
- ↑ "Crassulacean Acid Metabolism". Annual Review of Plant Physiology 36 (1): 595–622. 1985. doi:10.1146/annurev.pp.36.060185.003115. https://repository.arizona.edu/bitstream/10150/552219/1/dp_05_04-192.pdf.
- ↑ "Roots: evolutionary origins and biogeochemical significance". Journal of Experimental Botany 52 (Spec Issue): 381–401. March 2001. doi:10.1093/jexbot/52.suppl_1.381. PMID 11326045.
- ↑ "Ecophysiology of Crassulacean Acid Metabolism (CAM)". Annals of Botany 93 (6): 629–652. June 2004. doi:10.1093/aob/mch087. PMID 15150072.
- ↑ "Spatial resolution of an integrated C4+CAM photosynthetic metabolism". Science Advances 8 (31): eabn2349. August 2022. doi:10.1126/sciadv.abn2349. PMID 35930634. Bibcode: 2022SciA....8N2349M.
- ↑ "CAM Succulents". Physiological Ecology of North American Desert Plants. Adaptations of Desert Organisms. 1997. pp. 125–140. doi:10.1007/978-3-642-59212-6_6. ISBN 978-3-642-63900-5.
- ↑ Raven, P & Evert, R & Eichhorn, S, 2005, "Biology of Plants" (seventh edition), p. 135 (Figure 7-26), W.H. Freeman and Company Publishers ISBN:0-7167-1007-2
- ↑ 14.0 14.1 "CAM Photosynthesis in Submerged Aquatic Plants". Botanical Review 64 (2): 121–175. 1998. doi:10.1007/bf02856581.
- ↑ 15.0 15.1 "Carbon Assimilation Characteristics of the Aquatic CAM Plant, Isoetes howellii". Plant Physiology 76 (2): 525–530. October 1984. doi:10.1104/pp.76.2.525. PMID 16663874.
- ↑ "Seasonal variation in crassulacean acid metabolism by the aquatic isoetid Littorella uniflora". Photosynthesis Research 112 (3): 163–173. September 2012. doi:10.1007/s11120-012-9759-0. PMID 22766959.
- ↑ Wetland Ecology: Principles and Conservation. Cambridge, UK: Cambridge University Press. 2010. p. 26. ISBN 978-0521739672.
- ↑ "Crassulacean acid metabolism (CAM) supersedes the turgor loss point (TLP) as an important adaptation across a precipitation gradient, in the genus Clusia". Functional Plant Biology 48 (7): 703–716. June 2021. doi:10.1071/FP20268. PMID 33663679.
- ↑ "Physiological Changes in Portulacaria afra (L.) Jacq. during a Summer Drought and Rewatering". Plant Physiology 85 (2): 481–486. October 1987. doi:10.1104/pp.85.2.481. PMID 16665724.
- ↑ "Crassulacean Acid Metabolism in the Succulent C(4) Dicot, Portulaca oleracea L Under Natural Environmental Conditions". Plant Physiology 69 (4): 757–761. April 1982. doi:10.1104/pp.69.4.757. PMID 16662291.
- ↑ "Evolution of CAM and C4 Carbon-Concentrating Mechanisms". International Journal of Plant Sciences 164 (S3): S55–S77. 2003. doi:10.1086/374192. http://www.werc.usgs.gov/OLDsitedata/seki/pdfs/ijps_keeley_rundel.pdf.
- ↑ "Crassulacean acid metabolism: plastic, fantastic". Journal of Experimental Botany 53 (369): 569–580. April 2002. doi:10.1093/jexbot/53.369.569. PMID 11886877.
- ↑ "Underwater CAM photosynthesis elucidated by Isoetes genome". Nature Communications 12 (1): 6348. November 2021. doi:10.1038/s41467-021-26644-7. PMID 34732722. Bibcode: 2021NatCo..12.6348W.
- ↑ 24.0 24.1 "Evidence of crussulacean acid metabolism in two North American isoetids". Aquatic Botany 15 (4): 381–386. 1983. doi:10.1016/0304-3770(83)90006-2.
- ↑ "Degrees of crassulacean acid metabolism in tropical epiphytic and lithophytic ferns". Australian Journal of Plant Physiology 26 (8): 749. 1999. doi:10.1071/PP99001.
- ↑ "Diffusive Resistance, Titratable Acidity, and CO2 Fixation in Two Tropical Epiphytic Ferns". American Fern Journal 66 (4): 121–124. 1976. doi:10.2307/1546463. https://www.biodiversitylibrary.org/part/230393.
- ↑ 27.0 27.1 Crassulacean Acid Metabolism
- ↑ "abstract to Carter & Martin, The occurrence of Crassulacean acid metabolism among epiphytes in a high-rainfall region of Costa Rica, Selbyana 15(2): 104-106 (1994)". http://orton.catie.ac.cr/cgi-bin/wxis.exe/?IsisScript=OET.xis&method=post&formato=2&cantidad=1&expresion=mfn=013247.
- ↑ "The Occurrence of Crassulacean Acid Metabolism in Epiphytic Ferns, with an Emphasis on the Vittariaceae". International Journal of Plant Sciences 166 (4): 623–630. 2005. doi:10.1086/430334.
- ↑ "CAM-cycling in the cycad Dioon edule Lindl. in its natural tropical deciduous forest habitat in central Veracruz, Mexico". Botanical Journal of the Linnean Society 138 (2): 155–162. 2002. doi:10.1046/j.1095-8339.2002.138002155.x.
- ↑ "Environmental control of crassulacean acid metabolism in Welwitschia mirabilis Hook. Fil. in its range of natural distribution in the Namib desert". Oecologia 24 (4): 323–334. December 1976. doi:10.1007/BF00381138. PMID 28309109. Bibcode: 1976Oecol..24..323S.
- ↑ "Crassulacean Acid Metabolism and Crassulacean Acid Metabolism Modifications in Peperomia camptotricha". Plant Physiology 77 (1): 59–63. January 1985. doi:10.1104/pp.77.1.59. PMID 16664028.
- ↑ "Induction of Crassulacean Acid Metabolism in the Facultative Halophyte Mesembryanthemum crystallinum by Abscisic Acid". Plant Physiology 93 (3): 1253–1260. July 1990. doi:10.1104/pp.93.3.1253. PMID 16667587.
- ↑ "Leaf and Stem CO(2) Uptake in the Three Subfamilies of the Cactaceae". Plant Physiology 80 (4): 913–917. April 1986. doi:10.1104/pp.80.4.913. PMID 16664741.
- ↑ "Drought-stress-induced up-regulation of CAM in seedlings of a tropical cactus, Opuntia elatior, operating predominantly in the C3 mode". Journal of Experimental Botany 62 (11): 4037–4042. July 2011. doi:10.1093/jxb/err106. PMID 21504876.
- ↑ "The Occurrence and Phylogenetics of Crassulacean Acid Metabolism in the Portulacaceae". International Journal of Plant Sciences 162 (2): 257–262. 2001. doi:10.1086/319569.
- ↑ 37.0 37.1 37.2 37.3 37.4 37.5 37.6 "TANSLEY REVIEW No 1.: VARIATION IN PHOTOSYNTHETIC ACID METABOLISM IN VASCULAR PLANTS: CAM AND RELATED PHENOMENA". The New Phytologist 101 (1): 3–24. September 1985. doi:10.1111/j.1469-8137.1985.tb02815.x. PMID 33873823.
- ↑ 38.0 38.1 38.2 38.3 38.4 38.5 38.6 38.7 38.8 38.9 "Functional leaf anatomy of plants with crassulacean acid metabolism". Functional Plant Biology 32 (5): 409–419. July 2005. doi:10.1071/FP04195. PMID 32689143.
- ↑ 39.0 39.1 "Ecophysiology of Crassulacean Acid Metabolism (CAM)". Annals of Botany 93 (6): 629–652. June 2004. doi:10.1093/aob/mch087. PMID 15150072.
- ↑ 40.0 40.1 "C/C ratio changes in crassulacean Acid metabolism plants". Plant Physiology 52 (5): 427–430. November 1973. doi:10.1104/pp.52.5.427. PMID 16658576.
- ↑ 41.0 41.1 "The occurrence of Crassulacean Acid Metabolism a supplementary list during 1976 to 1979". Photosynthetica 13 (4): 467–473. 1979.
- ↑ "A phylogenetic view of low-level CAM in Pelargonium (Geraniaceae)". American Journal of Botany 90 (1): 135–142. January 2003. doi:10.3732/ajb.90.1.135. PMID 21659089.
- ↑ Crassulacean Acid Metabolism: Analysis of an Ecological Adaptation. de Ecological Studies. 30. Springer Science & Business Media. 2012. pp. 24. ISBN 978-3-642-67038-1.
- ↑ "Effect of Severe Water Stress on Aspects of Crassulacean Acid Metabolism in Xerosicyos". Plant Physiology 103 (4): 1089–1096. December 1993. doi:10.1104/pp.103.4.1089. PMID 12232003.
- ↑ Structure-Function Relations of Warm Desert Plants Adaptations of Desert Organisms. Springer Science & Business Media. 2012. pp. 118. ISBN 9783642609794.
- ↑ "Momordica charantia (bitter melon): 111016801". https://www.kegg.jp/dbget-bin/www_bget?mcha:111016801+mcha:111016802+mcha:111023897+mcha:111025911.
- ↑ "Plant Types: III. CAM Plants, Examples and Plant Families". 2013. https://www.cropsreview.com/cam-plants.html.
- ↑ 48.0 48.1 48.2 48.3 48.4 48.5 48.6 "Crassulacean Acid Metabolism 1975–2000, a Check List". Photosynthetica 39 (3): 339–352. 2001. doi:10.1023/A:1020292623960.
- ↑ 49.0 49.1 "The Ecological Water-Use Strategies of Succulent Plants". Advances in Botanical Research. 55. Academic Press. January 2010. pp. 179–225. https://www.brown.edu/Research/Edwards_Lab/reprints/ogburn_edwardsABR2010.pdf.
- ↑ "Prosopis juliflora". The Plant List. http://www.theplantlist.org/tpl1.1/record/ild-18491.
- ↑ "Variability in Crassulacean Acid Metabolism A Survey of North Carolina Succulent Species". Botanical Gazette 143 (4): 491–497. 1982. doi:10.1086/337326.
- ↑ "Crassulacean Acid Metabolism in the Gesneriaceae". American Journal of Botany 73 (3): 336–345. 1986. doi:10.1002/j.1537-2197.1986.tb12046.x. https://docs.rwu.edu/cgi/viewcontent.cgi?article=1290&context=fcas_fp.
- ↑ "Anatomy of Succulence and CAM in 15 Species of Senecio". Botanical Gazette 149 (2): 142–152. 1988. doi:10.1086/337701.
- ↑ "Crassulacean acid metabolism in the ZZ plant, Zamioculcas zamiifolia (Araceae)". American Journal of Botany 94 (10): 1670–1676. October 2007. doi:10.3732/ajb.94.10.1670. PMID 21636363.
- ↑ "Multiple origins of crassulacean acid metabolism and the epiphytic habit in the Neotropical family Bromeliaceae". Proceedings of the National Academy of Sciences of the United States of America 101 (10): 3703–3708. March 2004. doi:10.1073/pnas.0400366101. PMID 14982989. Bibcode: 2004PNAS..101.3703C.
- ↑ "Evolution along the crassulacean acid metabolism continuum.". Functional Plant Biology 37 (11): 995–1010. October 2010. doi:10.1071/FP10084. https://www.researchgate.net/publication/228660470.
External links
 |