Biology:Microbiome
A microbiome (from grc μικρός (mikrós) 'small', and βίος (bíos) 'life') is the community of microorganisms that can usually be found living together in any given habitat. It was defined more precisely in 1988 by Whipps et al. as "a characteristic microbial community occupying a reasonably well-defined habitat which has distinct physio-chemical properties. The term thus not only refers to the microorganisms involved but also encompasses their theatre of activity". In 2020, an international panel of experts published the outcome of their discussions on the definition of the microbiome. They proposed a definition of the microbiome based on a revival of the "compact, clear, and comprehensive description of the term" as originally provided by Whipps et al., but supplemented with two explanatory paragraphs. The first explanatory paragraph pronounces the dynamic character of the microbiome, and the second explanatory paragraph clearly separates the term microbiota from the term microbiome.
The microbiota consists of all living members forming the microbiome. Most microbiome researchers agree bacteria, archaea, fungi, algae, and small protists should be considered as members of the microbiome. The integration of phages, viruses, plasmids, and mobile genetic elements is more controversial. Whipps's "theatre of activity" includes the essential role secondary metabolites play in mediating complex interspecies interactions and ensuring survival in competitive environments. Quorum sensing induced by small molecules allows bacteria to control cooperative activities and adapts their phenotypes to the biotic environment, resulting, e.g., in cell-cell adhesion or biofilm formation.
All animals and plants form associations with microorganisms, including protists, bacteria, archaea, fungi, and viruses. In the ocean, animal–microbial relationships were historically explored in single host–symbiont systems. However, new explorations into the diversity of microorganisms associating with diverse marine animal hosts is moving the field into studies that address interactions between the animal host and the multi-member microbiome. The potential for microbiomes to influence the health, physiology, behaviour, and ecology of marine animals could alter current understandings of how marine animals adapt to change. This applies to especially the growing climate-related and anthropogenic-induced changes already impacting the ocean. The plant microbiome plays key roles in plant health and food production and has received significant attention in recent years. Plants live in association with diverse microbial consortia, referred to as the plant microbiota, living both inside (the endosphere) and outside (the episphere) of plant tissues. They play important roles in the ecology and physiology of plants. The core plant microbiome is thought to contain keystone microbial taxa essential for plant health and for the fitness of the plant holobiont. Likewise, the mammalian gut microbiome has emerged as a key regulator of host physiology, and coevolution between host and microbial lineages has played a key role in the adaptation of mammals to their diverse lifestyles.
Microbiome research originated in microbiology back in the seventeenth century. The development of new techniques and equipment boosted microbiological research and caused paradigm shifts in understanding health and disease.[1] The development of the first microscopes allowed the discovery of a new, unknown world and led to the identification of microorganisms. Infectious diseases became the earliest focus of interest and research. However, only a small proportion of microorganisms are associated with disease or pathogenicity. The overwhelming majority of microbes are essential for healthy ecosystem functioning and known for beneficial interactions with other microbes and organisms. The concept that microorganisms exist as single cells began to change as it became increasingly obvious that microbes occur within complex assemblages in which species interactions and communication are critical. Discovery of DNA, the development of sequencing technologies, PCR, and cloning techniques enabled the investigation of microbial communities using cultivation-independent approaches. Further paradigm shifts occurred at the beginning of this century and still continue, as new sequencing technologies and accumulated sequence data have highlighted both the ubiquity of microbial communities in association within higher organisms and the critical roles of microbes in human, animal, and plant health. These have revolutionised microbial ecology. The analysis of genomes and metagenomes in a high-throughput manner now provide highly effective methods for researching the functioning of both individual microorganisms as well as whole microbial communities in natural habitats.
Background
History
Microbiome research originated in microbiology and started back in the seventeenth century. The development of new techniques and equipment has boosted microbiological research and caused paradigm shifts in understanding health and disease. Since infectious diseases have affected human populations throughout most of history, medical microbiology was the earliest focus of research and public interest. Additionally, food microbiology is an old field of empirical applications. The development of the first microscopes allowed the discovery of a new, unknown world and led to the identification of microorganisms.[2]
- Paradigm shift
-
Shift of paradigm from microbes as unsocial organisms causing diseases to a holistic view of microorganisms as the centre of the One Health Concept interconnecting all areas of human lives.[2]
Access to the previously invisible world opened the eyes and the minds of the researchers of the seventeenth century. Antonie van Leeuwenhoek investigated diverse bacteria of various shapes, fungi, and protozoa, which he called animalcules, mainly from water, mud, and dental plaque samples, and discovered biofilms as a first indication of microorganisms interacting within complex communities. Robert Koch's explanation of the origin of human and animal diseases as a consequence of microbial infection and development of the concept of pathogenicity was an important milestone in microbiology. These findings shifted the focus of the research community and the public on the role of microorganisms as disease-forming agents that needed to be eliminated.[2]
However, comprehensive research over the past century has shown only a small proportion of microorganisms are associated with disease or pathogenicity. The overwhelming majority of microbes are essential for ecosystem functioning and known for beneficial interactions with other microbes as well as macroorganisms. In fact, maintaining a healthy microbiome is essential for human health and may be a target for new therapeutics.[3] At the end of the nineteenth century, microbial ecology started with the pioneering work by Martinus W. Beijerinck and Sergei Winogradsky. The newly established science of environmental microbiology resulted in another paradigm shift: microorganisms are everywhere in natural environments, often associated with hosts and, for the first time, beneficial effects on their hosts were reported.[4][5][2]
Subsequently, the concept that microorganisms exist as single cells began to change as it became increasingly obvious that microbes occur within complex assemblages in which species interactions and communication are critical to population dynamics and functional activities.[6] Discovery of DNA, the development of sequencing technologies, PCR, and cloning techniques enabled the investigation of microbial communities using cultivation-independent, DNA and RNA-based approaches.[7][2]
A further important step was the introduction of phylogenetic markers such as the 16S rRNA gene for microbial community analysis by Carl Woese and George E. Fox in 1977.[8] Nowadays biologists can barcode bacteria, archaea, fungi, algae, and protists in their natural habitats, e.g., by targeting their 16S and 18S rRNA genes, internal transcribed spacer (ITS), or, alternatively, specific functional regions of genes coding for specific enzymes.[9][10][11][2]
Another major paradigm shift was initiated at the beginning of this century and continues through today, as new sequencing technologies and accumulated sequence data have highlighted both the ubiquity of microbial communities in association within higher organisms and the critical roles of microbes in human, animal, and plant health.[12] These new possibilities have revolutionized microbial ecology, because the analysis of genomes and metagenomes in a high-throughput manner provides efficient methods for addressing the functional potential of individual microorganisms as well as of whole communities in their natural habitats.[13][14] Multiomics technologies including metatranscriptome, metaproteome and metabolome approaches now provide detailed information on microbial activities in the environment. Based on the rich foundation of data, the cultivation of microbes, which was often ignored or underestimated over the last thirty years, has gained new importance, and high throughput culturomics is now an important part of the toolbox to study microbiomes. The high potential and power of combining multiple "omics" techniques to analyze host-microbe interactions are highlighted in several reviews.[15][16][2]
Etymology
The word microbiome (from the Greek micro meaning "small" and bíos meaning "life") was first used by J.L. Mohr in 1952 in The Scientific Monthly to mean the microorganisms found in a specific environment.[58][59]
Definitions
Microbial communities have commonly been defined as the collection of microorganisms living together. More specifically, microbial communities are defined as multi-species assemblages, in which (micro) organisms interact with each other in a contiguous environment.[60] In 1988, Whipps and colleagues working on the ecology of rhizosphere microorganisms provided the first definition of the term microbiome.[61] They described the microbiome as a combination of the words micro and biome, naming a "characteristic microbial community" in a "reasonably well-defined habitat which has distinct physio-chemical properties" as their "theatre of activity". This definition represents a substantial advancement of the definition of a microbial community, as it defines a microbial community with distinct properties and functions and its interactions with its environment, resulting in the formation of specific ecological niches.[2]
However, many other microbiome definitions have been published in recent decades. By 2020 the most cited definition was by Lederberg,[62] and described microbiomes within an ecological context as a community of commensal, symbiotic, and pathogenic microorganisms within a body space or other environment. Marchesi and Ravel focused in their definition on the genomes and microbial (and viral) gene expression patterns and proteomes in a given environment and its prevailing biotic and abiotic conditions.[63] All these definitions imply that general concepts of macro-ecology could be easily applied to microbe-microbe as well as to microbe-host interactions. However, the extent to which these concepts, developed for macro-eukaryotes, can be applied to prokaryotes with their different lifestyles regarding dormancy, variation of phenotype, and horizontal gene transfer[64] as well as to micro-eukaryotes that is not quite clear. This raises the challenge of considering an entirely novel body of conceptual ecology models and theory for microbiome ecology, particularly in relation to the diverse hierarchies of interactions of microbes with one another and with the host biotic and abiotic environments. Many current definitions fail to capture this complexity and describe the term microbiome as encompassing the genomes of microorganisms only.[2]
| Microbiome definitions[2] | |
|---|---|
| Definition type | Examples |
| Ecological | Definitions based on ecology describe the microbiome following the concepts derived from the ecology of multicellular organisms. The main issue here is that the theories from the macro-ecology do not always fit the rules in the microbial world. |
| |
| Organisms/host-dependent | The host-dependent definitions are based on the microbial interactions with the host. The main gaps here concern the question whether the microbial-host interaction data gained from one host can be transferred to another. The understanding of coevolution and selection in the host-dependent definitions is also underrepresented. |
| |
| Genomic/ method-driven | There is a variety of microbiome definitions available that are driven by the methods applied. Mostly, these definitions rely on DNA sequence-based analysis and describe microbiome as a collective genome of microorganisms in a specific environment. The main bottleneck here is that every new available technology will result in a need for a new definition. |
| |
| Combined | There are some microbiome definitions available that fit several categories with their advantages and disadvantages. |
| |
In 2020, a panel of international experts, organised by the EU-funded MicrobiomeSupport project,[75] published the results of their deliberations on the definition of the microbiome.[2] The panel was composed of about 40 leaders from diverse microbiome areas, and about one hundred further experts from around the world contributed through an online survey. They proposed a definition of the microbiome based on a revival of what they characterised as the "compact, clear, and comprehensive description of the term" as originally provided by Whipps et al. in 1988,[61] amended with a set of recommendations considering subsequent technological developments and research findings. They clearly separate the terms microbiome and microbiota and provide a comprehensive discussion considering the composition of microbiota, the heterogeneity and dynamics of microbiomes in time and space, the stability and resilience of microbial networks, the definition of core microbiomes, and functionally relevant keystone species as well as co-evolutionary principles of microbe-host and inter-species interactions within the microbiome.[2]
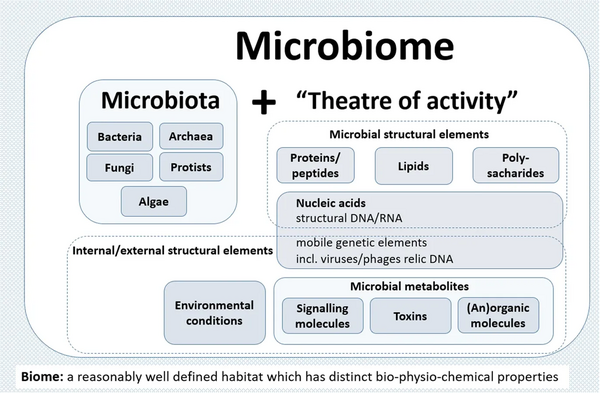
The panel extended the Whipps et al. definition, which contains all important points that are valid even 30 years after its publication in 1988, by two explanatory paragraphs differentiating the terms microbiome and microbiota and pronouncing its dynamic character, as follows:
- The microbiome is defined as a characteristic microbial community occupying a reasonable well-defined habitat which has distinct physio-chemical properties. The microbiome not only refers to the microorganisms involved but also encompass their theatre of activity, which results in the formation of specific ecological niches. The microbiome, which forms a dynamic and interactive micro-ecosystem prone to change in time and scale, is integrated in macro-ecosystems including eukaryotic hosts, and here crucial for their functioning and health.[2]
- The microbiota consists of the assembly of microorganisms belonging to different kingdoms (prokaryotes (bacteria, archaea), eukaryotes (algae, protozoa, fungi etc), while "their theatre of activity" includes microbial structures, metabolites, mobile genetic elements (such as transposons, phages, and viruses), and relic DNA embedded in the environmental conditions of the habitat.[2]
Membership
Microbiota
The microbiota comprises all living members forming the microbiome. Most microbiome researchers agree bacteria, archaea, fungi, algae, and small protists should be considered as members of the microbiome.[63][2] The integration of phages, viruses, plasmids, and mobile genetic elements is a more controversial issue in the definition of the microbiome. There is also no clear consensus as to whether extracellular DNA derived from dead cells, so-called "relic DNA", belongs to the microbiome.[76][2] Relic DNA can be up to 40% of the sequenced DNA in soil,[77] and was up to 33% of the total bacterial DNA on average in a broader analysis of habitats with the highest proportion of 80% in some samples.[78] Despite its omnipresence and abundance, relic DNA had a minimal effect on estimates of taxonomic and phylogenetic diversity.[78][2]
When it comes to the use of specific terms, a clear differentiation between microbiome and microbiota helps to avoid the controversy concerning the members of a microbiome.[2] Microbiota is usually defined as the assemblage of living microorganisms present in a defined environment.[63] As phages, viruses, plasmids, prions, viroids, and free DNA are usually not considered as living microorganisms,[79] they do not belong to the microbiota.[2]
The term microbiome, as it was originally postulated by Whipps and coworkers,[61] includes not only the community of the microorganisms but also their "theatre of activity". The latter involves the whole spectrum of molecules produced by the microorganisms, including their structural elements (nucleic acids, proteins, lipids, polysaccharides), metabolites (signalling molecules, toxins, organic, and inorganic molecules), and molecules produced by coexisting hosts and structured by the surrounding environmental conditions. Therefore, all mobile genetic elements, such as phages, viruses, and "relic" and extracellular DNA, should be included in the term microbiome, but are not a part of microbiota. The term microbiome is also sometimes confused with the metagenome. Metagenome is, however, clearly defined as a collection of genomes and genes from the members of a microbiota.[63][2]
Microbiome studies sometimes focus on the behaviour of a specific group of microbiota, generally in relation to or justified by a clear hypothesis. More and more terms like bacteriome, archaeome, mycobiome, or virome have started appearing in the scientific literature, but these terms do not refer to biomes (a regional ecosystem with a distinct assemblage of (micro) organisms, and physical environment often reflecting a certain climate and soil) as the microbiome itself.[2] Consequently, it would be better to use the original terms (bacterial, archaeal, or fungal community). In contrast to the microbiota, which can be studied separately, the microbiome is always composed by all members, which interact with each other, live in the same habitat, and form their ecological niche together. The well-established term virome is derived from virus and genome and is used to describe viral shotgun metagenomes consisting of a collection of nucleic acids associated with a particular ecosystem or holobiont.[80] Viral metagenomes can be suggested as a semantically and scientifically better term.[2]
Networks
-
Co-occurrence networks help visualising microbial interactions
Nodes usually represent taxa of microorganisms, and edges represent statistically significant associations between nodes.[2]
–––––––––––––––––––––––––––
Testing of the hypotheses resulted from the network analyses is required for a comprehensive study of microbial interactions.[2]
Microbes interact with one another, and these symbiotic interactions have diverse consequences for microbial fitness, population dynamics, and functional capacities within the microbiome.[81] The microbial interactions can either be between microorganisms of the same species or between different species, genera, families, and domains of life. The interactions can be separated into positive, negative, and neutral types. Positive interactions include mutualism, synergism, and commensalism. Negative interactions include amensalism, predation, parasitism, antagonism, and competition. Neutral interactions are interactions where there is no observed effect on the functional capacities or fitness of interacting species microbial life strategy concepts.[82]
Microbiomes exhibit different adaptive strategies.[2] Oligotrophs are organisms that can live in an environment offering very low levels of nutrients, particularly carbon. They are characterised by slow growth, low rates of metabolism, and generally low population density. Oligotrophic environments include deep oceanic sediments, caves, glacial and polar ice, deep subsurface soil, aquifers, ocean waters, and leached soils. In contrast are the copiotrophs, which thrive in much higher carbon concentrations, and do well in high organic substrate conditions such as sewage lagoons.[84][85]
In addition to oligotrophic and copiotrophic strategists, the competitor–stress tolerator–ruderals framework can influence the outcomes of interactions.[86] For example, microorganisms competing for the same source can also benefit from each other when competing for the same compound at different trophic levels. Stability of a complex microbial ecosystem depends on trophic interactions for the same substrate at different concentration levels. As of 2020 microbial social adaptations in nature have been understudied.[2] Here molecular markers can provide insight into social adaptations by supporting the theories, e.g., of altruists and cheaters in native microbiomes.[87][2]
Coevolution
- Shift in the understanding of the microbial-host coevolution
-
from "separation" theories to a holistic approachIn a holistic approach, the hosts and their associated microbiota are assumed to have coevolved with each other [2]
According to the "separation" approach, the microorganisms can be divided into pathogens, neutral, and symbionts, depending on their interaction with their host. The coevolution between host and its associated microbiota may be accordingly described as antagonistic (based on negative interactions) or mutualistic (based on positive interactions).[2][88]
As of 2020, the emergence in publications about opportunistic pathogens and pathobionts has produced a shift towards a holistic approach in the coevolutions theory. The holistic approach sees the host and its associated microbiota as one unit (the so-called holobiont), that coevolves as one entity. According to the holistic approach, holobiont's disease state is linked to dysbiosis, low diversity of the associated microbiota, and their variability: a so-called pathobiome state. The healthy state, on the other hand, is accompanied with eubiosis, high diversity, and uniformity of the respective microbiota.[2]
Types
Marine
- Marine animal host-microbiome relationship
-
Relationships are generally thought to exist in a symbiotic state, and are normally exposed to environmental and animal-specific factors that may cause natural variations. Some events may change the relationship into a functioning but altered symbiotic state, whereas extreme stress events may cause dysbiosis or a breakdown of the relationship and interactions.[89]
All animals on Earth form associations with microorganisms, including protists, bacteria, archaea, fungi, and viruses. In the ocean, animal–microbial relationships were historically explored in single host–symbiont systems. However, new explorations into the diversity of microorganisms associating with diverse marine animal hosts is moving the field into studies that address interactions between the animal host and a more multi-member microbiome. The potential for microbiomes to influence the health, physiology, behavior, and ecology of marine animals could alter current understandings of how marine animals adapt to change, and especially the growing climate-related and anthropogenic-induced changes already impacting the ocean environment.[89]
The microbiomes of diverse marine animals are currently under study, from simplistic organisms including sponges[90] and ctenophores [91] to more complex organisms such as sea squirts[92] and sharks.[93][89]
The relationship between the Hawaiian bobtail squid and the bioluminescent bacterium Aliivibrio fischeri is one of the best studied symbiotic relationships in the sea and is a choice system for general symbiosis research. This relationship has provided insight into fundamental processes in animal-microbial symbioses, and especially biochemical interactions and signaling between the host and bacterium.[94][95][89]
The gutless marine oligochaete worm Olavius algarvensis is another relatively well-studied marine host to microbes. These three centimetre long worms reside within shallow marine sediments of the Mediterranean Sea. The worms do not contain a mouth or a digestive or excretory system, but are instead nourished with the help of a suite of extracellular bacterial endosymbionts that reside upon coordinated use of sulfur present in the environment.[96] This system has benefited from some of the most sophisticated 'omics and visualization tools.[97] For example, multi-labeled probing has improved visualization of the microbiome[98] and transcriptomics and proteomics have been applied to examine host–microbiome interactions, including energy transfer between the host and microbes[99] and recognition of the consortia by the worm's innate immune system.[100] The major strength of this system is that it does offer the ability to study host–microbiome interactions with a low diversity microbial consortium, and it also offers a number of host and microbial genomic resources[97][101][89]

Corals are one of the more common examples of an animal host whose symbiosis with microalgae can turn to dysbiosis, and is visibly detected as bleaching. Coral microbiomes have been examined in a variety of studies, which demonstrate how variations in the ocean environment, most notably temperature, light, and inorganic nutrients, affect the abundance and performance of the microalgal symbionts, as well as calcification and physiology of the host.[103][104] Studies have also suggested that resident bacteria, archaea, and fungi additionally contribute to nutrient and organic matter cycling within the coral, with viruses also possibly playing a role in structuring the composition of these members, thus providing one of the first glimpses at a multi-domain marine animal symbiosis.[105] The gammaproteobacterium Endozoicomonas is emerging as a central member of the coral's microbiome, with flexibility in its lifestyle.[102][106] Given the recent mass bleaching occurring on reefs,[107] corals will likely continue to be a useful and popular system for symbiosis and dysbiosis research.[89]
Sponges are common members of the ocean's diverse benthic habitats and their abundance and ability to filter large volumes of seawater have led to the awareness that these organisms play critical roles in influencing benthic and pelagic processes in the ocean.[108] They are one of the oldest lineages of animals, and have a relatively simple body plan that commonly associates with bacteria, archaea, algal protists, fungi, and viruses.[109] Sponge microbiomes are composed of specialists and generalists, and complexity of their microbiome appears to be shaped by host phylogeny.[110] Studies have shown that the sponge microbiome contributes to nitrogen cycling in the oceans, especially through the oxidation of ammonia by archaea and bacteria.[111][112] Most recently, microbial symbionts of tropical sponges were shown to produce and store polyphosphate granules,[113] perhaps enabling the host to survive periods of phosphate depletion in oligotrophic marine environments.[114] The microbiomes of some sponge species do appear to change in community structure in response to changing environmental conditions, including temperature[115] and ocean acidification,[116][117] as well as synergistic impacts.[118]
-
Relative abundance of bacterial classes from whale blow, air and seawater samples.[120]
Cetacean microbiomes can be difficult to assess because of difficulties accessing microbial samples. For example, many whale species are rare and are deep divers. There are different techniques for sampling a cetacean's gut microbiome. The most common is collecting fecal samples from the environment and taking a probe from the center that is non-contaminated.[121] The skin is a barrier protecting marine mammals from the outside world. The epidermal microbiome on the skin is an indicator of how healthy the animal is, and is also an ecological indicator of the state of the surrounding environment. Knowing what the microbiome of the skin of marine mammals looks like under typical conditions allows understanding of how these communities different from free microbial communities found in the sea.[122] Cetaceans are in danger because they are affected by multiple stress factors which make them more vulnerable to various diseases. They have been high susceptibility to airway infections, but little is known about their respiratory microbiome. Sampling the exhaled breath or "blow" of cetaceans can provide an assessment of their state of health. Blow is composed of a mixture of microorganisms and organic material, including lipids, proteins , and cellular debris derived from the linings of the airways which, when released into the relatively cooler outdoor air, condense to form a visible mass of vapor, which can be collected. There are various methods for collecting exhaled breath samples, one of the most recent is through the use of aerial drones. This method provides a safer, quieter, and less invasive alternative and often a cost-effective option for monitoring fauna and flora. Blow samples are taken to the laboratory where the respiratory tract microbiota are amplified and sequenced. The use of aerial drones has been more successful with large cetaceans due to slow swim speeds and larger blow sizes.[123][124][119][125]
Terrestrial
Plant
-
Microbiomes in the plant ecosystem [126]
The plant microbiome plays roles in plant health and food production and has received significant attention in recent years.[127][128] Plants live in association with diverse microbial consortia. These microbes, referred to as the plant's microbiota, live both inside (the endosphere) and outside (the episphere) of plant tissues, and play important roles in the ecology and physiology of plants.[129] "The core plant microbiome is thought to comprise keystone microbial taxa that are important for plant fitness and established through evolutionary mechanisms of selection and enrichment of microbial taxa containing essential functions genes for the fitness of the plant holobiont."[130]
Plant microbiomes are shaped by both factors related to the plant itself, such as genotype, organ, species and health status, as well as factors related to the plant's environment, such as management, land use and climate.[131] The health status of a plant has been reported in some studies to be reflected by or linked to its microbiome.[132][127][133][128]
Plant and plant-associated microbiota colonise different niches on and inside the plant tissue. All the above-ground plant parts together, called the phyllosphere, are a continuously evolving habitat due to ultraviolet (UV) radiation and altering climatic conditions. It is primarily composed of leaves. Below-ground plant parts, mainly roots, are generally influenced by soil properties. Harmful interactions affect the plant growth through pathogenic activities of some microbiota members. On the other hand, beneficial microbial interactions promote plant growth.[126]
Animal
-
Principal coordinate analysis of animal gut microbiome data [134]
The mammalian gut microbiome has emerged as a key regulator of host physiology,[135] and coevolution between host and microbial lineages has played a key role in the adaptation of mammals to their diverse lifestyles. Diet, especially herbivory, is an important correlate of microbial diversity in mammals.[136][137] Most mammalian microbiomes are also strongly correlated with host phylogeny, despite profound shifts in diet.[136][138][139][140] This suggests host factors that themselves change across host phylogeny, such as gut physiology, play an important role in structuring the gut microbiomes across mammals. The vertebrate adaptive immune system is even speculated to have evolved as just such a factor for selective maintenance of symbiotic homeostasis.[141][134]
The importance of phylogeny-correlated factors to the diversity of vertebrate microbiomes more generally is still poorly understood. Phylosymbiosis, or the observation that more closely related host species have more similar microbiomes,[142][143] has been described in a number of nonmammalian taxa.[144][145] Other analyses have found substantial variation in phylosymbiotic signals among mammalian taxa,[146] sometimes with conflicting results.[147][148] The presence of a robust phylosymbiotic correlation implies that host factors control microbial assembly. Even if the specific mechanisms are unknown, variation in the strength or presence of a measurable phylosymbiotic signal across host phylogeny could prove useful for identifying such mechanisms through comparative studies. However, as of 2020 most studies have focused on just a few taxa at a time, and variable methods for both surveying the microbiome and measuring phylosymbiosis and host specificity (or the restriction of microbes to specific host lineages) have made generalisations difficult.[134]
Without broader evolutionary context, it is unclear how universally conserved patterns of host-microbe phylosymbiosis actually are. Growing evidence indicates that the strong patterns identified in mammals are the exception rather than the rule in vertebrates. Meta-analyses of fish [149] and birds [150] have failed to detect the strength of correlations to diet and phylogeny reported in mammals. A recent analysis of samples from more than 100 vertebrate species also found the strength of phylogenetic correlation to be much higher in mammals than in birds, reptiles, amphibians, or fish.[151] It is increasingly appreciated in nonvertebrate animals that fundamental aspects of the host's relationship to its symbiotic community can change drastically between taxa: many insects depend entirely on microbes for key metabolites, while others seem to be devoid of resident gut microbes.[152][134]
Human
The human microbiome is the aggregate of all microbiota that reside on or within human tissues and biofluids along with the corresponding anatomical sites in which they reside,[153] including the skin, mammary glands, seminal fluid, uterus, ovarian follicles, lung, saliva, oral mucosa, conjunctiva, biliary tract, and gastrointestinal tract. Types of human microbiota include bacteria, archaea, fungi, protists and viruses. Though micro-animals can also live on the human body, they are typically excluded from this definition. In the context of genomics, the term human microbiome is sometimes used to refer to the collective genomes of resident microorganisms;[154] the term human metagenome has the same meaning.[153]
Humans are colonised by many microorganisms, with approximately the same order of magnitude of non-human cells as human cells.[155] Some microorganisms that colonize humans are commensal, meaning they co-exist without harming or benefiting humans; others have a mutualistic relationship with their human hosts.[154]: 700 [156] Conversely, some non-pathogenic microorganisms can harm human hosts via the metabolites they produce, like trimethylamine, which the human body converts to trimethylamine N-oxide via FMO3-mediated oxidation.[157][158] Certain microorganisms perform tasks that are known to be useful to the human host, but the role of most of them is not well understood. Those that are expected to be present, and that under normal circumstances do not cause disease, are sometimes deemed normal flora or normal microbiota.[154]
The Human Microbiome Project (HMP) took on the project of sequencing the genome of the human microbiota, focusing particularly on the microbiota that normally inhabit the skin, mouth, nose, digestive tract, and vagina.[154] It reached a milestone in 2012 when it published its initial results.[159]
Assessment
Currently available methods for studying microbiomes, so-called multi-omics, range from high throughput isolation (culturomics) and visualization (microscopy), to targeting the taxonomic composition (metabarcoding), or addressing the metabolic potential (metabarcoding of functional genes, metagenomics) to analyze microbial activity (metatranscriptomics, metaproteomics, metabolomics). Based on metagenome data, microbial genomes can be reconstructed. While first metagenome-assembled genomes were reconstructed from environmental samples,[160] in recent years, several thousands of bacterial genomes were binned without culturing the organisms behind. For example, 154,723 microbial genomes of the global human microbiome were reconstructed in 2019 from 9,428 metagenomes.[161][2]
- Methods for assessing microbial functioning
-
Methods for assessing microbial functioningComplex microbiome studies cover various areas, starting from the level of complete microbial cells (microscopy, culturomics), followed by the DNA (single cell genomics, metabarcoding, metagenomics), RNA (metatranscriptomics), protein (metaproteomics), and metabolites (metabolomics). In that order, the focus of the studies shifts from the microbial potential (learning about available microbiota in the given habitat) over the metabolic potential (deciphering available genetic material) towards microbial functioning (e.g., the discovery of the active metabolic pathways).[2]
Computational modeling of microbiomes has been used to complement experimental methods for investigating microbial function by utilizing multi-omic data to predict complex inter-species and host-species dynamics.[162][163] A popular in silico method is to combine metabolic network models of microbial taxa present in a community and use a mathematical modeling strategy such as flux balance analysis to predict the metabolic function of the microbial community at a taxon and community-level.[164][165]
As of 2020, understanding remains limited due to missing links between the massive availability of microbiome DNA sequence data on the one hand and limited availability of microbial isolates needed to confirm metagenomic predictions of gene function on the other hand.[2] Metagenome data provides a playground for new predictions, yet much more data is needed to strengthen the links between sequence and rigorous functional predictions. This becomes obvious when considering that the replacement of one single amino acid residue by another may lead to a radical functional change, resulting in an incorrect functional assignment to a given gene sequence.[166] Additionally, cultivation of new strains is needed to help identify the large fraction of unknown sequences obtained from metagenomics analyses, which for poorly studied ecosystems can be more than 70%. Depending on the applied method, even in well-studied microbiomes, 40–70% of the annotated genes in fully sequenced microbial genomes have no known or predicted function.[167] As of 2019, 85 of the then established 118 phyla had not had a single species described, presenting a challenge to understanding prokaryotic functional diversity .[168][2]
The number of prokaryotic phyla may reach hundreds, and archaeal ones are among the least studied.[168] The growing gap between the diversity of Bacteria and Archaea held in pure culture and those detected by molecular methods has led to the proposal to establish a formal nomenclature for not-yet cultured taxa, primarily based on sequence information.[169][170] According to this proposal, the concept of Candidatus species would be extended to the groups of closely related genome sequences, and their names would be published following established rules of bacterial nomenclature.[2]
Each microbiome system is suited to address different types of questions based on the culturability of microbes, genetic tractability of microbes and host (where relevant), ability to maintain system in laboratory setting, and ability to make host/environment germfree.[171]
- Underlying complexity
-
Tradeoffs between experimental questions and complexity of microbiome systems [171]
(A) Pairwise interactions between the soil bacteria Bacillus subtilis and Streptomyces spp. are well-suited for characterizing the functions of secondary metabolites in microbial interactions.
(B) The symbiosis between bobtail squid and the marine bacterium Aliivibrio fischeri is fundamental to understanding host and microbial factors that influence colonization.
(C) The use of gnotobiotic mice is crucial for making links between host diet and the effects on specific microbial taxa in a community.[171]
See also
- Earth Microbiome Project
- Human microbiome
- Initial acquisition of microbiota
- Microbial population biology
- Microbiomes of the built environment
- Mycobiome
References
- ↑ Boctor, Joseph; Oweda, Mariam; El-Hadidi, Mohamed (2023), Mitra, Suparna, ed., "Comprehensive Guideline for Microbiome Analysis Using R" (in en), Metagenomic Data Analysis (New York, NY: Springer US) 2649: pp. 393–436, doi:10.1007/978-1-0716-3072-3_20, ISBN 978-1-0716-3071-6, https://link.springer.com/10.1007/978-1-0716-3072-3_20, retrieved 2023-11-24
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 2.23 2.24 2.25 2.26 2.27 2.28 2.29 2.30 2.31 2.32 2.33 2.34 2.35 2.36 Berg, Gabriele; Rybakova, Daria; Fischer, Doreen; Cernava, Tomislav et al. (2020). "Microbiome definition re-visited: Old concepts and new challenges". Microbiome 8 (1): 103. doi:10.1186/s40168-020-00875-0. PMID 32605663.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Merchak A, Gaultier A. Microbial metabolites and immune regulation: New targets for major depressive disorder. Brain Behav Immun Health. 2020 Nov 2;9:100169. doi: 10.1016/j.bbih.2020.100169. PMID 34589904; PMCID: PMC8474524.
- ↑ Hiltner L. (1902) "Die Keimungsverhältnisse der Leguminosensamen und ihre Beeinflussung durch Organismenwirkung". In: Parey P and Springer J (Eds.) Arb Biol Abt Land u Forstw K Gsndhtsamt, 3, Berlin. Pages 1–545.
- ↑ Metchnikoff E. The prolongation of life: optimistic studies. GP Putnam's Sons; 1908.
- ↑ Bassler, B.L. (2002) "Small talk: cell-to-cell communication in bacteria". Cell, 109(4): 421–424. doi:10.1016/S0092-8674(02)00749-3.
- ↑ Brul, S., Kallemeijn, W. and Smits, G. (2008) "Functional genomics for food microbiology: molecular mechanisms of weak organic-acid preservative adaptation in yeast". CAB Rev, 3: 1–14. doi:10.1079/PAVSNNR20083005.
- ↑ 8.0 8.1 Woese, C. R.; Fox, G. E. (1977). "Phylogenetic structure of the prokaryotic domain: The primary kingdoms". Proceedings of the National Academy of Sciences 74 (11): 5088–5090. doi:10.1073/pnas.74.11.5088. PMID 270744. Bibcode: 1977PNAS...74.5088W.
- ↑ Uksa, M., Schloter, M., Endesfelder, D., Kublik, S., Engel, M., Kautz, T., Köpke, U. and Fischer, D. (2015) "Prokaryotes in subsoil—evidence for a strong spatial separation of different phyla by analysing co-occurrence networks". Frontiers in microbiology, 6: 1269. doi:10.3389/fmicb.2015.01269.
- ↑ Maritz, J.M., Rogers, K.H., Rock, T.M., Liu, N., Joseph, S., Land, K.M. and Carlton, J.M. (2017) "An 18S rRNA workflow for characterizing protists in sewage, with a focus on zoonotic trichomonads". Microbial ecology, 74(4): 923–936. doi:10.1007/s00248-017-0996-9.
- ↑ Purahong, W., Wubet, T., Lentendu, G., Schloter, M., Pecyna, M.J., Kapturska, D., Hofrichter, M., Krüger, D. and Buscot, F. (2016) "Life in leaf litter: novel insights into community dynamics of bacteria and fungi during litter decomposition". Molecular Ecology, 25(16): 4059–4074. doi:10.1111/mec.13739.
- ↑ Lozupone, C.A., Stombaugh, J.I., Gordon, J.I., Jansson, J.K. and Knight, R. (2012) "Diversity, stability and resilience of the human gut microbiota". Nature, 489(7415): 220–230. doi:10.1038/nature11550.
- ↑ Venter, J.C., Remington, K., Heidelberg, J.F., Halpern, A.L., Rusch, D., Eisen, J.A., Wu, D., Paulsen, I., Nelson, K.E., Nelson, W. and Fouts, D.E. (2004) "Environmental genome shotgun sequencing of the Sargasso Sea". Science, 304(5667): 66–74. doi:10.1126/science.1093857.
- ↑ Liu, L., Li, Y., Li, S., Hu, N., He, Y., Pong, R., Lin, D., Lu, L. and Law, M. (2012) "Comparison of next-generation sequencing systems". BioMed Research International, 2012: 251364. doi:10.1155/2012/251364.
- ↑ Stegen, J.C., Bottos, E.M. and Jansson, J.K. (2018) "A unified conceptual framework for prediction and control of microbiomes". Current Opinion in Microbiology, 44: 20–27. doi:10.1016/j.mib.2018.06.002.
- ↑ Knight, R., Vrbanac, A., Taylor, B.C., Aksenov, A., Callewaert, C., Debelius, J., Gonzalez, A., Kosciolek, T., McCall, L.I., McDonald, D. and Melnik, A.V. (2018) "Best practices for analysing microbiomes". Nature Reviews Microbiology, 16(7): 410–422. doi:10.1038/s41579-018-0029-9.
- ↑ Lane, Nick (2015). "The unseen world: Reflections on Leeuwenhoek (1677) 'Concerning little animals'". Philosophical Transactions of the Royal Society B: Biological Sciences 370 (1666). doi:10.1098/rstb.2014.0344. PMID 25750239.
- ↑ Jarvis, Charles E. (2016). "Pier Antonio Micheli (1679–1737) and Carl Linnaeus (1707–1778)". Webbia 71: 1–24. doi:10.1080/00837792.2016.1147210.
- ↑ Riedel, Stefan (2005). "Edward Jenner and the History of Smallpox and Vaccination". Baylor University Medical Center Proceedings 18 (1): 21–25. doi:10.1080/08998280.2005.11928028. PMID 16200144.
- ↑ Martini, Alessandro (1993). "Origin and domestication of the wine yeast Saccharomyces cerevisiae". Journal of Wine Research 4 (3): 165–176. doi:10.1080/09571269308717966.
- ↑ Berche, P. (2012). "Louis Pasteur, from crystals of life to vaccination". Clinical Microbiology and Infection (Elsevier BV) 18: 1–6. doi:10.1111/j.1469-0691.2012.03945.x. ISSN 1198-743X. PMID 22882766.
- ↑ Evans, A.S. (1976) "Causation and disease: the Henle-Koch postulates revisited. The Yale journal of biology and medicine, 49(2): 175.
- ↑ Dworkin, Martin; Gutnick, David (2012). "Sergei Winogradsky: A founder of modern microbiology and the first microbial ecologist". FEMS Microbiology Reviews 36 (2): 364–379. doi:10.1111/j.1574-6976.2011.00299.x. PMID 22092289. http://conservancy.umn.edu/bitstream/11299/119577/1/WinoFEMSsubmitted.pdf.
- ↑ Hartmann, Anton; Rothballer, Michael; Schmid, Michael (2008). "Lorenz Hiltner, a pioneer in rhizosphere microbial ecology and soil bacteriology research". Plant and Soil 312 (1–2): 7–14. doi:10.1007/s11104-007-9514-z.
- ↑ "The Fluorescence Microscope". Microscopes—Help Scientists Explore Hidden Worlds. The Nobel Foundation. http://nobelprize.org/educational_games/physics/microscopes/fluorescence/.
- ↑ Borman, S., Russell, H. and Siuzdak, G., (2003) "A Mass Spec Timeline Developing techniques to measure mass has been a Nobel pursuit. Todays Chemist at Work, 12(9): 47–50.
- ↑ Waksman, Selman A. (1953). "Sergei Nikolaevitch Winogradsky: 1856-1953". Science 118 (3054): 36–37. doi:10.1126/science.118.3054.36. PMID 13076173. Bibcode: 1953Sci...118...36W.
- ↑ Griffith, Fred (1928). "The Significance of Pneumococcal Types". Journal of Hygiene 27 (2): 113–159. doi:10.1017/S0022172400031879. PMID 20474956.
- ↑ Hayes, W. (1966) "Genetic Transformation: a Retrospective Appreciation", First Griffith Memorial Lecture. Microbiology, 45(3): 385–397.
- ↑ American Chemical Society (1999) Discovery and Development of Penicillin, 1928–1945. International Historic Chemical Landmarks, The Alexander Fleming Laboratory Museum, London.
- ↑ Ruska, Ernst (1987). "The Development of the Electron Microscope and of Electron Microscopy(Nobel Lecture)". Angewandte Chemie International Edition in English 26 (7): 595–605. doi:10.1002/anie.198705953.
- ↑ Avery, O. T.; MacLeod, C. M.; McCarty, M. (1979). "Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Inductions of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III". Journal of Experimental Medicine 149 (2): 297–326. doi:10.1084/jem.149.2.297. PMID 33226.
- ↑ o'Malley, Maureen A. (2018). "The Experimental Study of Bacterial Evolution and Its Implications for the Modern Synthesis of Evolutionary Biology". Journal of the History of Biology 51 (2): 319–354. doi:10.1007/s10739-017-9493-8. PMID 28980196.
- ↑ Rich, Alexander (2003). "The double helix: A tale of two puckers". Nature Structural & Molecular Biology 10 (4): 247–249. doi:10.1038/nsb0403-247. PMID 12660721.
- ↑ Cassidy, Andrew; Jones, Julia (2014). "Developments in in situ hybridisation". Methods 70 (1): 39–45. doi:10.1016/j.ymeth.2014.04.006. PMID 24747923.
- ↑ Crick, Francis (1970). "Central Dogma of Molecular Biology". Nature 227 (5258): 561–563. doi:10.1038/227561a0. PMID 4913914. Bibcode: 1970Natur.227..561C.
- ↑ Meyer, Veronika (2013). Practical high-performance liquid chromatography. Hoboken, N.J: Wiley. ISBN 978-1-118-68134-3. OCLC 864917338. https://books.google.com/books?id=ODRYwLsJy3AC.
- ↑ Grunstein, M.; Hogness, D. S. (1975). "Colony hybridization: A method for the isolation of cloned DNAs that contain a specific gene". Proceedings of the National Academy of Sciences 72 (10): 3961–3965. doi:10.1073/pnas.72.10.3961. PMID 1105573. Bibcode: 1975PNAS...72.3961G.
- ↑ Sanger, F.; Nicklen, S.; Coulson, A. R. (1977). "DNA sequencing with chain-terminating inhibitors". Proceedings of the National Academy of Sciences 74 (12): 5463–5467. doi:10.1073/pnas.74.12.5463. PMID 271968. Bibcode: 1977PNAS...74.5463S.
- ↑ Heather, James M.; Chain, Benjamin (2016). "The sequence of sequencers: The history of sequencing DNA". Genomics 107 (1): 1–8. doi:10.1016/j.ygeno.2015.11.003. PMID 26554401. PMC 4727787. https://discovery.ucl.ac.uk/1475547/1/1-s2.0-S0888754315300410-main.pdf.
- ↑ Eme, Laura; Spang, Anja; Lombard, Jonathan; Stairs, Courtney W.; Ettema, Thijs J. G. (2017). "Archaea and the origin of eukaryotes". Nature Reviews Microbiology 15 (12): 711–723. doi:10.1038/nrmicro.2017.133. PMID 29123225.
- ↑ Fiers, W.; Contreras, R.; Duerinck, F.; Haegeman, G.; Iserentant, D.; Merregaert, J.; Min Jou, W.; Molemans, F. et al. (1976). "Complete nucleotide sequence of bacteriophage MS2 RNA: Primary and secondary structure of the replicase gene". Nature 260 (5551): 500–507. doi:10.1038/260500a0. PMID 1264203. Bibcode: 1976Natur.260..500F.
- ↑ Prusiner, Stanley B. (1982). "Novel Proteinaceous Infectious Particles Cause Scrapie". Science 216 (4542): 136–144. doi:10.1126/science.6801762. PMID 6801762. Bibcode: 1982Sci...216..136P.
- ↑ Mullis, K.B. (1990) "The unusual origin of the polymerase chain reaction". Scientific American, 262(4): 56–65.
- ↑ Higuchi, Russell; Fockler, Carita; Dollinger, Gavin; Watson, Robert (1993). "Kinetic PCR Analysis: Real-time Monitoring of DNA Amplification Reactions". Nature Biotechnology 11 (9): 1026–1030. doi:10.1038/nbt0993-1026. PMID 7764001.
- ↑ Bentleylawrence, J.; Villnave, C. A.; Singer, R. H. (1988). "Sensitive, high-resolution chromatin and chromosome mapping in situ: Presence and orientation of two closely integrated copies of EBV in a lymphoma line". Cell 52 (1): 51–61. doi:10.1016/0092-8674(88)90530-2. PMID 2830981.
- ↑ Huber, D.; Voith von Voithenberg, L.; Kaigala, G.V. (2018). "Fluorescence in situ hybridization (FISH): History, limitations and what to expect from micro-scale FISH?". Micro and Nano Engineering (Elsevier BV) 1: 15–24. doi:10.1016/j.mne.2018.10.006. ISSN 2590-0072.
- ↑ Zhang, Tong; Fang, Herbert H. P. (2006). "Applications of real-time polymerase chain reaction for quantification of microorganisms in environmental samples". Applied Microbiology and Biotechnology (Springer Science and Business Media LLC) 70 (3): 281–289. doi:10.1007/s00253-006-0333-6. ISSN 0175-7598. PMID 16470363.
- ↑ Flemming, Hans-Curt (1993). "Biofilms and Environmental Protection". Water Science and Technology 27 (7–8): 1–10. doi:10.2166/wst.1993.0528.
- ↑ Flemming (2011). Biofilm highlights. Heidelberg New York: Springer-Verlag Berlin Heidelberg. ISBN 978-3-642-19940-0. OCLC 769756150. https://books.google.com/books?id=1azigYOg8woC.
- ↑ Amann, R. I.; Ludwig, W.; Schleifer, K. H. (1995). "Phylogenetic identification and in situ detection of individual microbial cells without cultivation". Microbiological Reviews 59 (1): 143–169. doi:10.1128/mr.59.1.143-169.1995. PMID 7535888.
- ↑ Fleischmann, Robert D. et al. (1995). "Whole-Genome Random Sequencing and Assembly of Haemophilus influenzae Rd". Science 269 (5223): 496–512. doi:10.1126/science.7542800. PMID 7542800. Bibcode: 1995Sci...269..496F.
- ↑ Kulski, Jerzy K. (2016). "Next-Generation Sequencing — an Overview of the History, Tools, and "Omic" Applications". Next Generation Sequencing - Advances, Applications and Challenges. doi:10.5772/61964. ISBN 978-953-51-2240-1. https://research-repository.uwa.edu.au/en/publications/nextgeneration-sequencing--an-overview-of-the-history-tools-and-omic-applications(08c0444b-aa1c-4b50-a93b-e9e945cc75e5).html.
- ↑ Stern, A.; Mick, E.; Tirosh, I.; Sagy, O.; Sorek, R. (2012). "CRISPR targeting reveals a reservoir of common phages associated with the human gut microbiome". Genome Research 22 (10): 1985–1994. doi:10.1101/gr.138297.112. PMID 22732228.
- ↑ Schadt, E. E.; Turner, S.; Kasarskis, A. (2010). "A window into third-generation sequencing". Human Molecular Genetics 19 (R2): R227–R240. doi:10.1093/hmg/ddq416. PMID 20858600.
- ↑ Vogel, Timothy M.; Simonet, Pascal; Jansson, Janet K.; Hirsch, Penny R.; Tiedje, James M.; Van Elsas, Jan Dirk; Bailey, Mark J.; Nalin, Renaud et al. (2009). "Terra Genome: A consortium for the sequencing of a soil metagenome". Nature Reviews Microbiology 7 (4): 252. doi:10.1038/nrmicro2119.
- ↑ Gilbert, Jack A.; Meyer, Folker; Jansson, Janet; Gordon, Jeff; Pace, Norman; Tiedje, James; Ley, Ruth; Fierer, Noah et al. (2010). "The Earth Microbiome Project: Meeting report of the "1st EMP meeting on sample selection and acquisition" at Argonne National Laboratory October 6th 2010". Standards in Genomic Sciences 3 (3): 249–253. doi:10.4056/aigs.1443528. PMID 21304728.
- ↑ "BioConcepts". http://www.biological-concepts.com/views/search.php?term=1442.
- ↑ microbiome (3rd ed.), Oxford University Press, September 2005, http://www.oed.com/view/Entry/365863, retrieved 2020-12-18 (Subscription or UK public library membership required.)
- ↑ Konopka, A. (2009) "What is microbial community ecology?" The ISME Journal, 3(11): 1223–1230. Konopka, A., 2009. What is microbial community ecology?. The ISME journal, 3(11), pp.1223–1230. doi:10.1038/ismej.2009.88.
- ↑ 61.0 61.1 61.2 61.3 Whipps J., Lewis K. and Cooke R. (1988) "Mycoparasitism and plant disease control". In: Burge M (Ed.) Fungi in Biological Control Systems, Manchester University Press, pages 161–187. ISBN 9780719019791.
- ↑ 62.0 62.1 Lederberg, J. and McCray, A.T. (2001) "'Ome Sweet'Omics—A genealogical treasury of words". The Scientist, 15(7): 8.
- ↑ 63.0 63.1 63.2 63.3 63.4 Marchesi, J.R. and Ravel, J. (2015) "The vocabulary of microbiome research: a proposal". Microbiome, 3(31). doi:10.1186/s40168-015-0094-5.
- ↑ Prosser, J.I., Bohannan, B.J., Curtis, T.P., Ellis, R.J., Firestone, M.K., Freckleton, R.P., Green, J.L., Green, L.E., Killham, K., Lennon, J.J. and Osborn, A.M. (2007) "The role of ecological theory in microbial ecology". Nature Reviews Microbiology, 5(5): 384–392. doi:10.1038/nrmicro1643.
- ↑ del Carmen Orozco-Mosqueda, M., del Carmen Rocha-Granados, M., Glick, B.R. and Santoyo, G. (2018) "Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms". Microbiological Research, 208: 25–31. doi:10.1016/j.micres.2018.01.005.
- ↑ 66.0 66.1 Merriam-Webster Dictionary – microbiome.
- ↑ Human Microbiome Project. Accessed 25 Aug 2020.
- ↑ Nature.com: Microbiome. Accessed 25 August 2020.
- ↑ ScienceDirect: Microbiome Accessed 25 August 2020.
- ↑ Arevalo, P., VanInsberghe, D., Elsherbini, J., Gore, J. and Polz, M.F. (2019) "A reverse ecology approach based on a biological definition of microbial populations". Cell, 178(4): 820–834. doi:10.1016/j.cell.2019.06.033.
- ↑ Schlaeppi, K. and Bulgarelli, D. (2015) "The plant microbiome at work". Molecular Plant-Microbe Interactions, 28(3): 212–217. doi:10.1094/MPMI-10-14-0334-FI.
- ↑ Rogers Y-H and Zhang C. (2016) "Genomic Technologies in Medicine and Health: Past, Present, and Future". In: Kumar D and Antonarakis S. (Eds.) Medical and Health Genomics. Oxford: Academic Press, pages 15–28. ISBN 9780127999227.
- ↑ Ho, H.E. and Bunyavanich, S. (2018) "Role of the microbiome in food allergy". Current allergy and asthma reports, 18(4): 27. doi:10.1007/s11882-018-0780-z.
- ↑ Whiteside, S.A., Razvi, H., Dave, S., Reid, G. and Burton and J.P. (2015) "The microbiome of the urinary tract—a role beyond infection". Nature Reviews Urology, 12(2): 81–90. doi:10.1038/nrurol.2014.361.
- ↑ MicrobiomeSupport project
- ↑ Carini, Paul (2016) A census of the dead: the story behind microbial 'relic DNA' in soil Nature Research: Microbiology.
- ↑ Carini, P., Marsden, P.J., Leff, J.W., Morgan, E.E., Strickland, M.S. and Fierer, N. (2016) "Relic DNA is abundant in soil and obscures estimates of soil microbial diversity". Nature Microbiology, 2(3): 1–6. doi:10.1038/nmicrobiol.2016.242.
- ↑ 78.0 78.1 Lennon, J.T., Muscarella, M.E., Placella, S.A. and Lehmkuhl, B.K. (2018) "How, when, and where relic DNA affects microbial diversity". mBio, 9(3). doi:10.1128/mBio.00637-18.
- ↑ Dupré JO, O'Malley MA (2009) "Varieties of living things: life at the intersection of lineage and metabolism". In: Normandin S and Wolfe C (Eds.) Vitalism and the Scientific Image in Post-Enlightenment Life Science 1800–2010. Dordrecht: Springer, pages 311–344. Lua error: not enough memory..
- ↑ McDaniel, L., Breitbart, M., Mobberley, J., Long, A., Haynes, M., Rohwer, F. and Paul, J.H., 2008. Metagenomic analysis of lysogeny in Tampa Bay: implications for prophage gene expression. PLoS One, 3(9), p.e3263. doi:10.1371/journal.pone.0003263.
- ↑ Banerjee, Samiran; Schlaeppi, Klaus; Van Der Heijden, Marcel G. A. (2018). "Keystone taxa as drivers of microbiome structure and functioning". Nature Reviews Microbiology 16 (9): 567–576. doi:10.1038/s41579-018-0024-1. PMID 29789680. https://www.zora.uzh.ch/id/eprint/168373/8/Banerjee_et_al_Revised_NRM_Manuscript.pdf.
- ↑ Kern, Lara; Abdeen, Suhaib K; Kolodziejczyk, Aleksandra A; Elinav, Eran (October 2021). "Commensal inter-bacterial interactions shaping the microbiota". Current Opinion in Microbiology 63: 158–171. doi:10.1016/j.mib.2021.07.011. PMID 34365152.
- ↑ Riera, Joan Lluís; Baldo, Laura (29 September 2020). "Microbial co-occurrence networks of gut microbiota reveal community conservation and diet-associated shifts in cichlid fishes". Animal Microbiome (Springer Science and Business Media LLC) 2 (1): 36. doi:10.1186/s42523-020-00054-4. ISSN 2524-4671. PMID 33499972.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Koch, Arthur L. (2001). "Oligotrophs versus copiotrophs". BioEssays (Wiley) 23 (7): 657–661. doi:10.1002/bies.1091. ISSN 0265-9247. PMID 11462219.
- ↑ Ho, Adrian; Lonardo, D. Paolo Di; Bodelier, Paul L. E. (22 January 2017). "Revisiting life strategy concepts in environmental microbial ecology". FEMS Microbiology Ecology (Oxford University Press (OUP)) 93 (3): fix006. doi:10.1093/femsec/fix006. ISSN 1574-6941. PMID 28115400.
- ↑ Ho, Adrian; Lonardo, D. Paolo Di; Bodelier, Paul L. E. (2017). "Revisiting life strategy concepts in environmental microbial ecology". FEMS Microbiology Ecology 93 (3): fix006. doi:10.1093/femsec/fix006. PMID 28115400.
- ↑ Banerjee, Samiran; Schlaeppi, Klaus; Van Der Heijden, Marcel G. A. (2018). "Keystone taxa as drivers of microbiome structure and functioning". Nature Reviews Microbiology 16 (9): 567–576. doi:10.1038/s41579-018-0024-1. PMID 29789680. https://www.zora.uzh.ch/id/eprint/168373/8/Banerjee_et_al_Revised_NRM_Manuscript.pdf.
- ↑ Leftwich, Philip T.; Edgington, Matthew P.; Chapman, Tracey (2020-09-09). "Transmission efficiency drives host–microbe associations" (in en). Proceedings of the Royal Society B: Biological Sciences 287 (1934): 20200820. doi:10.1098/rspb.2020.0820. ISSN 0962-8452. PMID 32873208.
- ↑ 89.0 89.1 89.2 89.3 89.4 89.5 Apprill, A. (2017) "Marine animal microbiomes: toward understanding host–microbiome interactions in a changing ocean". Frontiers in Marine Science, 4: 222. doi:10.3389/fmars.2017.00222.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Webster, N.S., Negri, A.P., Botté, E.S., Laffy, P.W., Flores, F., Noonan, S., Schmidt, C. and Uthicke, S. (2016) "Host-associated coral reef microbes respond to the cumulative pressures of ocean warming and ocean acidification". Scientific reports, 6: 19324. doi:10.1038/srep19324.
- ↑ Daniels, C. and Breitbart, M. (2012) "Bacterial communities associated with the ctenophores Mnemiopsis leidyi and Beroe ovata". FEMS Microbiology Ecology, 82(1): 90–101. doi:10.1111/j.1574-6941.2012.01409.x.
- ↑ Blasiak, L.C., Zinder, S.H., Buckley, D.H. and Hill, R.T. (2014) "Bacterial diversity associated with the tunic of the model chordate Ciona intestinalis". The ISME Journal, 8(2): 309–320. doi:10.1038/ismej.2013.156.
- ↑ Givens, C.E., Ransom, B., Bano, N. and Hollibaugh, J.T. (2015) "Comparison of the gut microbiomes of 12 bony fish and 3 shark species". Marine Ecology Progress Series, 518: 209–223. doi:10.3354/meps11034.
- ↑ McFall-Ngai, M.J. (2000) "Negotiations between animals and bacteria: the 'diplomacy'of the squid-vibrio symbiosis". Comparative Biochemistry and Physiology, Part A: Molecular & Integrative Physiology, 126(4): 471–480. doi:10.1016/S1095-6433(00)00233-6.
- ↑ McFall-Ngai, M. (2014) "Divining the essence of symbiosis: insights from the squid-vibrio model". PLoS Biology, 12(2): e1001783. doi:10.1371/journal.pbio.1001783.
- ↑ Dubilier, N., Mülders, C., Ferdelman, T., de Beer, D., Pernthaler, A., Klein, M., Wagner, M., Erséus, C., Thiermann, F., Krieger, J. and Giere, O. (2001) "Endosymbiotic sulphate-reducing and sulphide-oxidizing bacteria in an oligochaete worm". Nature, 411(6835): 298–302. doi:10.1038/35077067.
- ↑ 97.0 97.1 Woyke, T., Teeling, H., Ivanova, N.N., Huntemann, M., Richter, M., Gloeckner, F.O., Boffelli, D., Anderson, I.J., Barry, K.W., Shapiro, H.J. and Szeto, E. (2006) "Symbiosis insights through metagenomic analysis of a microbial consortium". Nature, 443(7114): 950–955. doi:10.1038/nature05192.
- ↑ Schimak, M.P., Kleiner, M., Wetzel, S., Liebeke, M., Dubilier, N. and Fuchs, B.M. (2016) "MiL-FISH: Multilabeled oligonucleotides for fluorescence in situ hybridization improve visualization of bacterial cells". Applied and Environmental Microbiology, 82(1): 62–70. doi:10.1128/AEM.02776-15.
- ↑ Kleiner, M., Wentrup, C., Lott, C., Teeling, H., Wetzel, S., Young, J., Chang, Y.J., Shah, M., VerBerkmoes, N.C., Zarzycki, J. and Fuchs, G. (2012) "Metaproteomics of a gutless marine worm and its symbiotic microbial community reveal unusual pathways for carbon and energy use". Proceedings of the National Academy of Sciences, 109(19): E1173–E1182. doi:10.1073/pnas.1121198109.
- ↑ Wippler, J., Kleiner, M., Lott, C., Gruhl, A., Abraham, P.E., Giannone, R.J., Young, J.C., Hettich, R.L. and Dubilier, N. (2016) "Transcriptomic and proteomic insights into innate immunity and adaptations to a symbiotic lifestyle in the gutless marine worm Olavius algarvensis". BMC Genomics, 17(1): 942. doi:10.1186/s12864-016-3293-y.
- ↑ Ruehland, C., Blazejak, A., Lott, C., Loy, A., Erséus, C. and Dubilier, N. (2008) "Multiple bacterial symbionts in two species of co‐occurring gutless oligochaete worms from Mediterranean sea grass sediments". Environmental microbiology, 10(12): 3404–3416. doi:10.1111/j.1462-2920.2008.01728.x.
- ↑ 102.0 102.1 Neave, M.J., Apprill, A., Ferrier-Pagès, C. and Voolstra, C.R. (2016) "Diversity and function of prevalent symbiotic marine bacteria in the genus Endozoicomonas". Applied Microbiology and Biotechnology, 100(19): 8315–8324. doi:10.1007/s00253-016-7777-0.
- ↑ Dubinsky, Z. and Jokiel, P.L. (1994) "Ratio of energy and nutrient fluxes regulates symbiosis between zooxanthellae and corals". Pacific Science, 48(3): 313–324.
- ↑ Anthony, K.R., Kline, D.I., Diaz-Pulido, G., Dove, S. and Hoegh-Guldberg, O.(2008) "Ocean acidification causes bleaching and productivity loss in coral reef builders". Proceedings of the National Academy of Sciences, 105(45): 17442–17446. doi:10.1073/pnas.0804478105.
- ↑ Bourne, D.G., Morrow, K.M. and Webster, N.S. (2016) "Insights into the coral microbiome: underpinning the health and resilience of reef ecosystems". Annual Review of Microbiology, 70: 317–340. doi:10.1146/annurev-micro-102215-095440.
- ↑ Neave, M.J., Michell, C.T., Apprill, A. and Voolstra, C.R. (2017) "Endozoicomonas genomes reveal functional adaptation and plasticity in bacterial strains symbiotically associated with diverse marine hosts". Scientific Reports, 7: 40579. doi:10.1038/srep40579.
- ↑ Hughes, T.P., Kerry, J.T., Álvarez-Noriega, M., Álvarez-Romero, J.G., Anderson, K.D., Baird, A.H., Babcock, R.C., Beger, M., Bellwood, D.R., Berkelmans, R. and Bridge, T.C. (2017) "Global warming and recurrent mass bleaching of corals". Nature, 543(7645): 373–377. doi:10.1038/nature21707.
- ↑ Bell, J.J. (2008) "The functional roles of marine sponges". Estuarine, Coastal and Shelf Science, 79(3): 341–353. doi:10.1016/j.ecss.2008.05.002.
- ↑ Webster, N.S. and Thomas, T. (2016) "The sponge hologenome". mBio, 7(2). doi:10.1128/mBio.00135-16.
- ↑ Thomas, T., Moitinho-Silva, L., Lurgi, M., Björk, J.R., Easson, C., Astudillo-García, C., Olson, J.B., Erwin, P.M., López-Legentil, S., Luter, H. and Chaves-Fonnegra, A. (2016) "Diversity, structure and convergent evolution of the global sponge microbiome". Nature Communications, 7(1): 1–12. doi:10.1038/ncomms11870.
- ↑ Bayer, K., Schmitt, S. and Hentschel, U. (2008) "Physiology, phylogeny and in situ evidence for bacterial and archaeal nitrifiers in the marine sponge Aplysina aerophoba". Environmental Microbiology, 10(11): 2942–2955. doi:10.1111/j.1462-2920.2008.01582.x.
- ↑ Radax, R., Hoffmann, F., Rapp, H.T., Leininger, S. and Schleper, C. (2012) "Ammonia‐oxidizing archaea as main drivers of nitrification in cold‐water sponges". Environmental Microbiology, 14(4): 909_923. doi:10.1111/j.1462-2920.2011.02661.x.
- ↑ Zhang, F., Blasiak, L.C., Karolin, J.O., Powell, R.J., Geddes, C.D. and Hill, R.T. (2015) "Phosphorus sequestration in the form of polyphosphate by microbial symbionts in marine sponges". Proceedings of the National Academy of Sciences, 112(14): 4381–4386. doi:10.1073/pnas.1423768112.
- ↑ Colman, A.S. (2015) "Sponge symbionts and the marine P cycle". Proceedings of the National Academy of Sciences, 112(14): 4191–4192. doi:10.1073/pnas.1502763112.
- ↑ Simister, R., Taylor, M.W., Tsai, P., Fan, L., Bruxner, T.J., Crowe, M.L. and Webster, N. (2012) "Thermal stress responses in the bacterial biosphere of the Great Barrier Reef sponge, Rhopaloeides odorabile. Environmental Microbiology, 14(12): 3232–3246. doi:10.1111/1462-2920.12010.
- ↑ Morrow, K.M., Bourne, D.G., Humphrey, C., Botté, E.S., Laffy, P., Zaneveld, J., Uthicke, S., Fabricius, K.E. and Webster, N.S. (2015) "Natural volcanic CO 2 seeps reveal future trajectories for host–microbial associations in corals and sponges". The ISME Journal, 9(4): 894–908. doi:10.1038/ismej.2014.188.
- ↑ Ribes, M., Calvo, E., Movilla, J., Logares, R., Coma, R. and Pelejero, C. (2016) "Restructuring of the sponge microbiome favors tolerance to ocean acidification. Environmental Microbiology Reports, 8(4): 536–544. doi:10.1111/1758-2229.12430.
- ↑ Lesser, M.P., Fiore, C., Slattery, M. and Zaneveld, J. (2016) "Climate change stressors destabilize the microbiome of the Caribbean barrel sponge, Xestospongia muta". Journal of Experimental Marine Biology and Ecology, 475: 11–18. doi:10.1016/j.jembe.2015.11.004.
- ↑ 119.0 119.1 "A novel non‐invasive tool for disease surveillance of free‐ranging whales and its relevance to conservation programs.". Animal Conservation 13 (2): 217–225. April 2010. doi:10.1111/j.1469-1795.2009.00326.x.
- ↑ "An economical custom-built drone for assessing whale health.". Frontiers in Marine Science 4: 425. December 2017. doi:10.3389/fmars.2017.00425.
- ↑ "Fecal microbiota of captive Antillean manatee Trichechus manatus manatus". FEMS Microbiology Letters 366 (11). June 2019. doi:10.1093/femsle/fnz134. PMID 31210263.
- ↑ "Humpback whales harbour a combination of specific and variable skin bacteria". Environmental Microbiology Reports 3 (2): 223–232. April 2011. doi:10.1111/j.1758-2229.2010.00213.x. PMID 23761254.
- ↑ "Interannual comparison of core taxa and community composition of the blow microbiota from East Australian humpback whales". FEMS Microbiology Ecology 95 (8). August 2019. doi:10.1093/femsec/fiz102. PMID 31260051.
- ↑ "The use of Unmanned Aerial Vehicles (UAVs) to sample the blow microbiome of small cetaceans". PLOS ONE 15 (7): e0235537. 2020. doi:10.1371/journal.pone.0235537. PMID 32614926. Bibcode: 2020PLoSO..1535537C.
- ↑ "Virological Sampling of Inaccessible Wildlife with Drones". Viruses 10 (6): 300. June 2018. doi:10.3390/v10060300. PMID 29865228.
- ↑ 126.0 126.1 Shelake, R.M., Pramanik, D. and Kim, J.Y. (2019) "Exploration of plant-microbe interactions for sustainable agriculture in CRISPR era". Microorganisms, 7(8): 269. doi:10.3390/microorganisms7080269.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ 127.0 127.1 Turner, Thomas R.; James, Euan K.; Poole, Philip S. (2013). "The plant microbiome". Genome Biology 14 (6): 209. doi:10.1186/gb-2013-14-6-209. PMID 23805896.
- ↑ 128.0 128.1 Purahong, Witoon; Orrù, Luigi; Donati, Irene; Perpetuini, Giorgia; Cellini, Antonio; Lamontanara, Antonella; Michelotti, Vania; Tacconi, Gianni et al. (2018). "Plant Microbiome and Its Link to Plant Health: Host Species, Organs and Pseudomonas syringae pv. Actinidiae Infection Shaping Bacterial Phyllosphere Communities of Kiwifruit Plants". Frontiers in Plant Science 9: 1563. doi:10.3389/fpls.2018.01563. PMID 30464766..
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Dastogeer, K.M., Tumpa, F.H., Sultana, A., Akter, M.A. and Chakraborty, A. (2020) "Plant microbiome–an account of the factors that shape community composition and diversity". Current Plant Biology: 100161. doi:10.1016/j.cpb.2020.100161.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Compant, S., Samad, A., Faist, H. and Sessitsch, A. (2019) "A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application". Journal of advanced research, 19: 29_37.doi:10.1016/j.jare.2019.03.004.
- ↑ Bringel, Franã§Oise; Couã©e, Ivan (2015). "Pivotal roles of phyllosphere microorganisms at the interface between plant functioning and atmospheric trace gas dynamics". Frontiers in Microbiology 06: 486. doi:10.3389/fmicb.2015.00486. PMID 26052316.
- ↑ Berendsen, Roeland L.; Pieterse, Corné M.J.; Bakker, Peter A.H.M. (2012). "The rhizosphere microbiome and plant health". Trends in Plant Science 17 (8): 478–486. doi:10.1016/j.tplants.2012.04.001. PMID 22564542.
- ↑ Berg, Gabriele; Grube, M.; Schloter, M.; Smalla, K. (2014). "The plant microbiome and its importance for plant and human health". Frontiers in Microbiology 5: 491. doi:10.3389/fmicb.2014.00491. PMID 25278934.
- ↑ 134.0 134.1 134.2 134.3 Song, Se Jin; Sanders, Jon G.; Delsuc, Frédéric; Metcalf, Jessica et al. (2020). "Comparative Analyses of Vertebrate Gut Microbiomes Reveal Convergence between Birds and Bats". mBio 11 (1). doi:10.1128/mBio.02901-19. PMID 31911491.
 Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Modified text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ McFall-Ngai, Margaret; Hadfield, Michael G.; Bosch, Thomas C. G.; Carey, Hannah V. et al. (2013). "Animals in a bacterial world, a new imperative for the life sciences". Proceedings of the National Academy of Sciences 110 (9): 3229–3236. doi:10.1073/pnas.1218525110. PMID 23391737. Bibcode: 2013PNAS..110.3229M.
- ↑ 136.0 136.1 Ley, Ruth E.; Hamady, Micah; Lozupone, Catherine; Turnbaugh, Peter J.; Ramey, Rob Roy; Bircher, J. Stephen; Schlegel, Michael L.; Tucker, Tammy A. et al. (2008). "Evolution of Mammals and Their Gut Microbes". Science 320 (5883): 1647–1651. doi:10.1126/science.1155725. PMID 18497261. Bibcode: 2008Sci...320.1647L.
- ↑ Muegge, Brian D.; Kuczynski, Justin; Knights, Dan; Clemente, Jose C.; González, Antonio; Fontana, Luigi; Henrissat, Bernard; Knight, Rob et al. (2011). "Diet Drives Convergence in Gut Microbiome Functions Across Mammalian Phylogeny and within Humans". Science 332 (6032): 970–974. doi:10.1126/science.1198719. PMID 21596990. Bibcode: 2011Sci...332..970M.
- ↑ Amato, Katherine R.; g. Sanders, Jon; Song, Se Jin; Nute, Michael; Metcalf, Jessica L.; Thompson, Luke R.; Morton, James T.; Amir, Amnon et al. (2019). "Evolutionary trends in host physiology outweigh dietary niche in structuring primate gut microbiomes". The ISME Journal 13 (3): 576–587. doi:10.1038/s41396-018-0175-0. PMID 29995839.
- ↑ Groussin, Mathieu; Mazel, Florent; Sanders, Jon G.; Smillie, Chris S.; Lavergne, Sébastien; Thuiller, Wilfried; Alm, Eric J. (2017). "Unraveling the processes shaping mammalian gut microbiomes over evolutionary time". Nature Communications 8: 14319. doi:10.1038/ncomms14319. PMID 28230052. Bibcode: 2017NatCo...814319G.
- ↑ Sanders, Jon G.; Beichman, Annabel C.; Roman, Joe; Scott, Jarrod J.; Emerson, David; McCarthy, James J.; Girguis, Peter R. (2015). "Baleen whales host a unique gut microbiome with similarities to both carnivores and herbivores". Nature Communications 6: 8285. doi:10.1038/ncomms9285. PMID 26393325. Bibcode: 2015NatCo...6.8285S.
- ↑ McFall-Ngai, Margaret (2007). "Care for the community". Nature 445 (7124): 153. doi:10.1038/445153a. PMID 17215830.
- ↑ Brucker, Robert M.; Bordenstein, Seth R. (2012). "The Roles of Host Evolutionary Relationships (Genus: Nasonia) and Development in Structuring Microbial Communities". Evolution 66 (2): 349–362. doi:10.1111/j.1558-5646.2011.01454.x. PMID 22276533.
- ↑ Brooks, Andrew W.; Kohl, Kevin D.; Brucker, Robert M.; Van Opstal, Edward J.; Bordenstein, Seth R. (2016). "Phylosymbiosis: Relationships and Functional Effects of Microbial Communities across Host Evolutionary History". PLOS Biology 14 (11): e2000225. doi:10.1371/journal.pbio.2000225. PMID 27861590.
- ↑ Sanders, Jon G.; Powell, Scott; Kronauer, Daniel J. C.; Vasconcelos, Heraldo L.; Frederickson, Megan E.; Pierce, Naomi E. (2014). "Stability and phylogenetic correlation in gut microbiota: Lessons from ants and apes". Molecular Ecology 23 (6): 1268–1283. doi:10.1111/mec.12611. PMID 24304129.
- ↑ Pollock, F. Joseph; McMinds, Ryan; Smith, Styles; Bourne, David G.; Willis, Bette L.; Medina, Mónica; Thurber, Rebecca Vega; Zaneveld, Jesse R. (2018). "Coral-associated bacteria demonstrate phylosymbiosis and cophylogeny". Nature Communications 9 (1): 4921. doi:10.1038/s41467-018-07275-x. PMID 30467310. Bibcode: 2018NatCo...9.4921P.
- ↑ Nishida, Alex H.; Ochman, Howard (2018). "Rates of gut microbiome divergence in mammals". Molecular Ecology 27 (8): 1884–1897. doi:10.1111/mec.14473. PMID 29290090.
- ↑ Phillips, Caleb D.; Phelan, Georgina; Dowd, Scot E.; McDonough, Molly M. et al. (2012). "Microbiome analysis among bats describes influences of host phylogeny, life history, physiology and geography". Molecular Ecology 21 (11): 2617–2627. doi:10.1111/j.1365-294X.2012.05568.x. PMID 22519571.
- ↑ Carrillo-Araujo, Mario; Taåÿ, Neslihan; Alcã¡Ntara-Hernã¡Ndez, Rocio J.; Gaona, Osiris; Schondube, Jorge E.; Medellãn, Rodrigo A.; Jansson, Janet K.; Falcã³n, Luisa I. (2015). "Phyllostomid bat microbiome composition is associated to host phylogeny and feeding strategies". Frontiers in Microbiology 6: 447. doi:10.3389/fmicb.2015.00447. PMID 26042099.
- ↑ Sullam, Karen E.; Essinger, Steven D.; Lozupone, Catherine A.; o'Connor, Michael P.; Rosen, Gail L.; Knight, ROB; Kilham, Susan S.; Russell, Jacob A. (2012). "Environmental and ecological factors that shape the gut bacterial communities of fish: A meta-analysis". Molecular Ecology 21 (13): 3363–3378. doi:10.1111/j.1365-294X.2012.05552.x. PMID 22486918.
- ↑ Waite, David W.; Taylor, Michael W. (2015). "Exploring the avian gut microbiota: Current trends and future directions". Frontiers in Microbiology 6: 673. doi:10.3389/fmicb.2015.00673. PMID 26191057.
- ↑ Youngblut, Nicholas D.; Reischer, Georg H.; Walters, William; Schuster, Nathalie; Walzer, Chris; Stalder, Gabrielle; Ley, Ruth E.; Farnleitner, Andreas H. (2019). "Host diet and evolutionary history explain different aspects of gut microbiome diversity among vertebrate clades". Nature Communications 10 (1): 2200. doi:10.1038/s41467-019-10191-3. PMID 31097702. Bibcode: 2019NatCo..10.2200Y.
- ↑ Hammer, Tobin J.; Sanders, Jon G.; Fierer, Noah (2019). "Not all animals need a microbiome". FEMS Microbiology Letters 366 (10). doi:10.1093/femsle/fnz117. PMID 31132110.
- ↑ 153.0 153.1 "The vocabulary of microbiome research: a proposal". Microbiome 3: 31. 2015. doi:10.1186/s40168-015-0094-5. PMID 26229597. "
Microbiome
This term refers to the entire habitat, including the microorganisms (bacteria, archaea, lower and higher eurkaryotes, and viruses), their genomes (i.e., genes), and the surrounding environmental conditions. This definition is based on that of “biome,” the biotic and abiotic factors of given environments. Others in the field limit the definition of microbiome to the collection of genes and genomes of members of a microbiota. It is argued that this is the definition of metagenome, which combined with the environment constitutes the microbiome.". - ↑ 154.0 154.1 154.2 154.3 Prescott's Microbiology (9th ed.). New York: McGraw Hill. 2013. pp. 713–721. ISBN 9780073402406. OCLC 886600661. https://books.google.com/books?id=sBCSRAAACAAJ.
- ↑ "Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans". Cell 164 (3): 337–40. January 2016. doi:10.1016/j.cell.2016.01.013. PMID 26824647.
- ↑ "Gut bacteria in health and disease". Gastroenterology & Hepatology 9 (9): 560–9. September 2013. PMID 24729765.
- ↑ "Microbiology Meets Big Data: The Case of Gut Microbiota-Derived Trimethylamine". Annual Review of Microbiology 69: 305–21. 2015. doi:10.1146/annurev-micro-091014-104422. PMID 26274026. "we review literature on trimethylamine (TMA), a microbiota-generated metabolite linked to atherosclerosis development.".
- ↑ "Archaea and the human gut: new beginning of an old story". World Journal of Gastroenterology 20 (43): 16062–78. November 2014. doi:10.3748/wjg.v20.i43.16062. PMID 25473158. "Trimethylamine is exclusively a microbiota-derived product of nutrients (lecithin, choline, TMAO, L-carnitine) from normal diet, from which seems originate two diseases, trimethylaminuria (or Fish-Odor Syndrome) and cardiovascular disease through the proatherogenic property of its oxidized liver-derived form.".
- ↑ "NIH Human Microbiome Project defines normal bacterial makeup of the body". NIH News. 13 June 2012. http://www.nih.gov/news/health/jun2012/nhgri-13.htm.
- ↑ Anantharaman, Karthik; Brown, Christopher T.; Hug, Laura A.; Sharon, Itai et al. (2016). "Thousands of microbial genomes shed light on interconnected biogeochemical processes in an aquifer system". Nature Communications 7: 13219. doi:10.1038/ncomms13219. PMID 27774985. Bibcode: 2016NatCo...713219A.
- ↑ Pasolli, Edoardo; Asnicar, Francesco; Manara, Serena; Zolfo, Moreno et al. (2019). "Extensive Unexplored Human Microbiome Diversity Revealed by over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle". Cell 176 (3): 649–662.e20. doi:10.1016/j.cell.2019.01.001. PMID 30661755.
- ↑ Kumar, Manish; Ji, Boyang; Zengler, Karsten; Nielsen, Jens (2019-07-23). "Modelling approaches for studying the microbiome". Nature Microbiology 4 (8): 1253–1267. doi:10.1038/s41564-019-0491-9. ISSN 2058-5276. PMID 31337891. http://dx.doi.org/10.1038/s41564-019-0491-9.
- ↑ Borenstein, Elhanan (May 15, 2012). "Computational systems biology and in silico modeling of the human microbiome". Briefings in Bioinformatics 13 (6): 769–780. doi:10.1093/bib/bbs022. PMID 22589385. https://doi.org/10.1093/bib/bbs022.
- ↑ Colarusso, Analeigha V.; Goodchild-Michelman, Isabella; Rayle, Maya; Zomorrodi, Ali R. (2021-06-04). "Computational modeling of metabolism in microbial communities on a genome-scale". Current Opinion in Systems Biology 26: 46–57. doi:10.1016/j.coisb.2021.04.001. ISSN 2452-3100.
- ↑ Biggs, Matthew B.; Medlock, Gregory L.; Kolling, Glynis L.; Papin, Jason A. (2015-06-24). "Metabolic network modeling of microbial communities". Wiley Interdisciplinary Reviews: Systems Biology and Medicine 7 (5): 317–334. doi:10.1002/wsbm.1308. ISSN 1939-5094. PMID 26109480. PMC 4575871. http://dx.doi.org/10.1002/wsbm.1308.
- ↑ Bloom, J. D.; Arnold, F. H. (2009). "In the light of directed evolution: Pathways of adaptive protein evolution". Proceedings of the National Academy of Sciences 106 (Suppl 1): 9995–10000. doi:10.1073/pnas.0901522106. PMID 19528653.
- ↑ Heintz-Buschart, Anna; Wilmes, Paul (2018). "Human Gut Microbiome: Function Matters". Trends in Microbiology 26 (7): 563–574. doi:10.1016/j.tim.2017.11.002. PMID 29173869.
- ↑ 168.0 168.1 Overmann, Jörg; Huang, Sixing; Nübel, Ulrich; Hahnke, Richard L.; Tindall, Brian J. (2019). "Relevance of phenotypic information for the taxonomy of not-yet-cultured microorganisms". Systematic and Applied Microbiology 42 (1): 22–29. doi:10.1016/j.syapm.2018.08.009. PMID 30197212.
- ↑ Konstantinidis, Konstantinos T.; Rosselló-Móra, Ramon; Amann, Rudolf (2017). "Uncultivated microbes in need of their own taxonomy". The ISME Journal 11 (11): 2399–2406. doi:10.1038/ismej.2017.113. PMID 28731467.
- ↑ Chuvochina, Maria; Rinke, Christian; Parks, Donovan H.; Rappé, Michael S.; Tyson, Gene W.; Yilmaz, Pelin; Whitman, William B.; Hugenholtz, Philip (2019). "The importance of designating type material for uncultured taxa". Systematic and Applied Microbiology 42 (1): 15–21. doi:10.1016/j.syapm.2018.07.003. PMID 30098831.
- ↑ 171.0 171.1 171.2 Chevrette, M.G., Bratburd, J.R., Currie, C.R. and Stubbendieck, R.M. (2019 "Experimental Microbiomes: Models Not to Scale". mSystems, 4(4): e00175-19. doi:10.1128/mSystems.00175-19.
Lua error: Internal error: The interpreter exited with status 1.
External links
Lua error: Internal error: The interpreter exited with status 1.
Lua error: Internal error: The interpreter exited with status 1.
![Shift of paradigm from microbes as unsocial organisms causing diseases to a holistic view of microorganisms as the centre of the One Health Concept interconnecting all areas of human lives.[2]](/wiki/images/3/33/Microbiome_paradigm_shifts.png)
![Co-occurrence networks help visualising microbial interactions Nodes usually represent taxa of microorganisms, and edges represent statistically significant associations between nodes.[2] ––––––––––––––––––––––––––– Testing of the hypotheses resulted from the network analyses is required for a comprehensive study of microbial interactions.[2]](/wiki/images/thumb/2/21/Microbial_interactions_visualized_through_microbial_co-occurrence_networks.webp/615px-Microbial_interactions_visualized_through_microbial_co-occurrence_networks.webp.png)
![Co-occurrence networks show difference in gut microbiota between herbivorous and carnivorous cichlids Nodes coloured according to phylum. The herbivore network has higher complexity (156 nodes and 339 edges) compared to the carnivore network (21 nodes and 70 edges).[83]](/wiki/images/thumb/d/d0/Co-occurrence_networks_showing_difference_in_gut_microbiota_between_herbivorous_and_carnivorous_cichlids.webp/563px-Co-occurrence_networks_showing_difference_in_gut_microbiota_between_herbivorous_and_carnivorous_cichlids.webp.png)
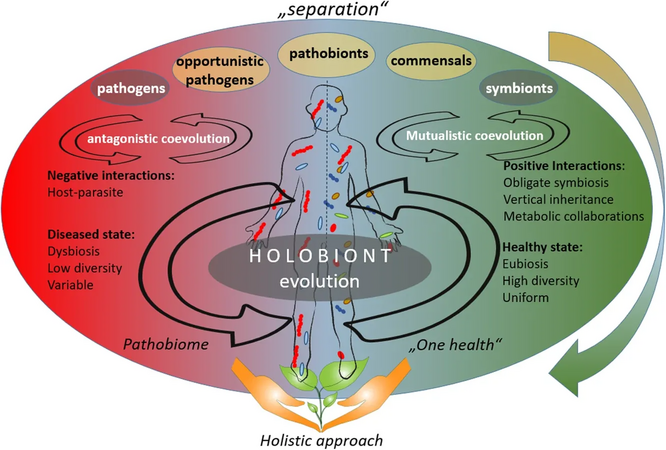
![Relationships are generally thought to exist in a symbiotic state, and are normally exposed to environmental and animal-specific factors that may cause natural variations. Some events may change the relationship into a functioning but altered symbiotic state, whereas extreme stress events may cause dysbiosis or a breakdown of the relationship and interactions.[89]](/wiki/images/b/be/Marine_animal_host-microbiome_relationships.jpg)

![Relative abundance of bacterial classes from whale blow, air and seawater samples.[120]](/wiki/images/thumb/b/b5/Cetacean_blow%27s_bacteria.png/426px-Cetacean_blow%27s_bacteria.png)
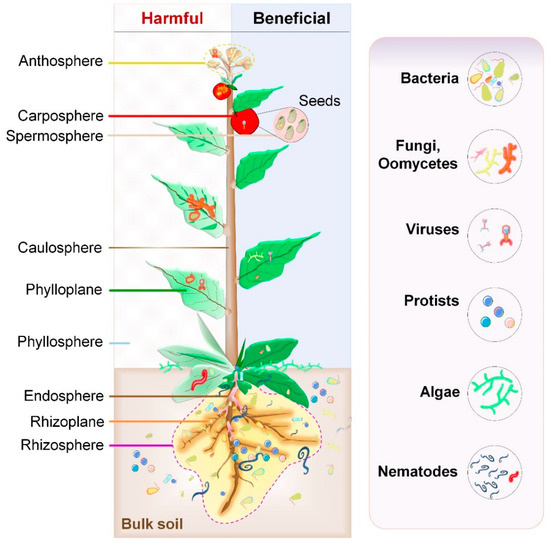
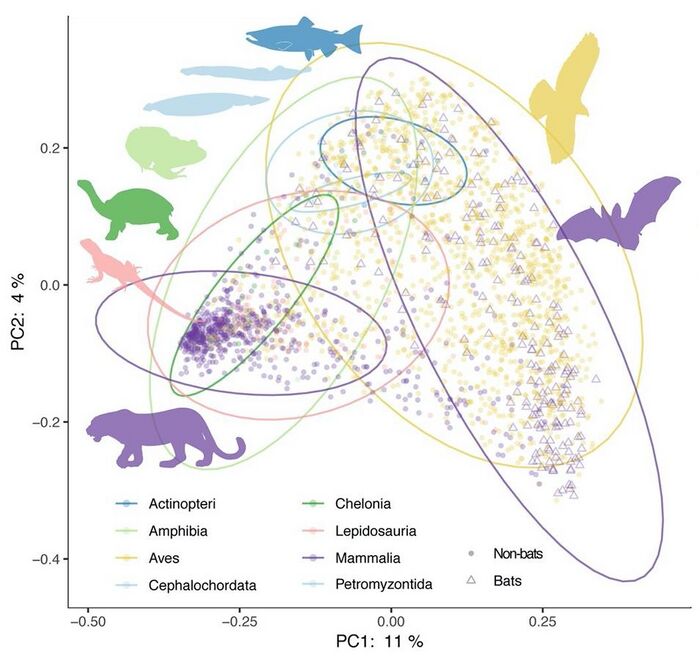
![Methods for assessing microbial functioning Complex microbiome studies cover various areas, starting from the level of complete microbial cells (microscopy, culturomics), followed by the DNA (single cell genomics, metabarcoding, metagenomics), RNA (metatranscriptomics), protein (metaproteomics), and metabolites (metabolomics). In that order, the focus of the studies shifts from the microbial potential (learning about available microbiota in the given habitat) over the metabolic potential (deciphering available genetic material) towards microbial functioning (e.g., the discovery of the active metabolic pathways).[2]](/wiki/images/thumb/1/13/Methods_for_assessing_microbial_functioning.webp/972px-Methods_for_assessing_microbial_functioning.webp.png)
![Tradeoffs between experimental questions and complexity of microbiome systems [171] (A) Pairwise interactions between the soil bacteria Bacillus subtilis and Streptomyces spp. are well-suited for characterizing the functions of secondary metabolites in microbial interactions. (B) The symbiosis between bobtail squid and the marine bacterium Aliivibrio fischeri is fundamental to understanding host and microbial factors that influence colonization. (C) The use of gnotobiotic mice is crucial for making links between host diet and the effects on specific microbial taxa in a community.[171]](/wiki/images/thumb/0/03/Tradeoffs_between_experimental_questions_and_complexity_of_microbiome_systems.jpg/1096px-Tradeoffs_between_experimental_questions_and_complexity_of_microbiome_systems.jpg)