Biology:Linaprazan
From HandWiki
Short description: Pharmaceutical molecule
 | |
| Clinical data | |
|---|---|
| Other names | AZD-0865 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
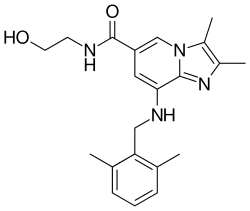
| Formula | C21H26N4O2 |
| Molar mass | 366.465 g·mol−1 |
Linaprazan is an experimental drug for the treatment of gastroesophageal reflux disease (GERD). Unlike the proton-pump inhibitors (PPIs) which are typically used to treat GERD, linaprazan is a potassium-competitive acid blocker (P-CAB).[1][2] Linaprazan was developed by AstraZeneca, but it was not successful in clinical trials.[3]
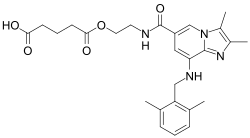
The drug was then licensed to Cinclus Pharma,[4] which is now investigating linaprazan glurate, a prodrug of linaprazan which is expected to have a longer biological half-life than linaprazan itself.[4]
References
- ↑ "Potassium-competitive acid blockers - are they the next generation of proton pump inhibitors?". World Journal of Gastrointestinal Pharmacology and Therapeutics 9 (7): 63–68. December 2018. doi:10.4292/wjgpt.v9.i7.63. PMID 30595950.
- ↑ "Linaprazan". Inxight Drugs. National Center for Advancing Translational Sciences. https://drugs.ncats.io/drug/E0OU4SC8DP.
- ↑ "Can reformulation of an AstraZeneca castoff rival Takeda's new heartburn drug? Here's a $26M bet on yes". 4 March 2020. https://endpts.com/can-reformulation-of-an-astrazeneca-castoff-rival-takedas-new-heartburn-drug-heres-a-26m-bet-on-yes/.
- ↑ 4.0 4.1 "Linaprazan glurate". Cinclus Pharma. https://cincluspharma.com/our-science/linaprazan-glurate/.
 |