Chemistry:4-Vinyltoluene
From HandWiki
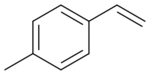
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Ethenyl-4-methylbenzene | |
| Other names
1-Methyl-4-vinylbenzene
4-Methylstyrene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
| MeSH | C042272 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H10 | |
| Molar mass | 118.179 g·mol−1 |
| Appearance | colorless liquid |
| Boiling point | 170–175 °C (338–347 °F; 443–448 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
4-Vinyltoluene is an organic compound with the formula CH3C6H4CH=CH2. It is derivative of styrene and is used as a comonomer in the production of specialized polystyrenes. It is produced by the dehydrogenation of 4-ethyltoluene.[1] It is also sometimes used in the production of styrene-free Polyester resin.
References
- ↑ Denis H. James; William M. Castor (2007), "Styrene", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 1, doi:10.1002/14356007.a25_329.pub2
 |

