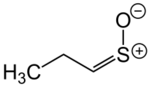
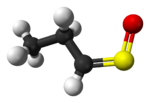
Chemistry:Syn-Propanethial-S-oxide
| |
| |
| Names | |
|---|---|
| IUPAC name
(Z)-propylidene-λ4-sulfanone[1]
| |
| Other names
Thiopropanal S-oxide
1-Sulfinylpropane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H6OS | |
| Molar mass | 90.14 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
syn-Propanethial S-oxide (or (Z)-propanethial S-oxide), a member of a class of organosulfur compounds known as thiocarbonyl S-oxides (formerly "sulfines"),[2] is a volatile liquid that acts as a lachrymatory agent (triggers tearing and stinging on contact with the eyes). The chemical is released from onions, Allium cepa, as they are sliced. The release is due to the breaking open of the onion cells and their releasing enzymes called alliinases, which then break down amino acid sulfoxides, generating sulfenic acids. A specific sulfenic acid, 1-propenesulfenic acid, formed when onions are cut, is rapidly rearranged by a second enzyme, called the lachrymatory factor synthase or LFS, giving syn-propanethial S-oxide.[3] The gas diffuses through the air and, on contact with the eye, it stimulates sensory neurons creating a stinging, painful sensation. Tears are released from the tear glands to dilute and flush out the irritant.[4] A structurally related lachrymatory compound, syn-butanethial S-oxide, C4H8OS, has been found in another genus Allium plant, Allium siculum.[5]
See also
References
- ↑ IUPAC Chemical Nomenclature and Structure Representation Division (2013). "P-74.2.2.1.8". in Favre, Henri A.; Powell, Warren H.. Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. IUPAC–RSC. ISBN 978-0-85404-182-4. https://pubs.rsc.org/en/Content/eBook/978-0-85404-182-4.
- ↑ Zwanenburg, B. (2004). "Thioaldehyde and Thioketone S-Oxides and S-Imides (Sulfines and Derivatives)". in Padwa, A.. Heteroatom Analogues of Aldehydes and Ketones. Science of Synthesis. 27. pp. 135–176. ISBN 978-1-58890-204-7.
- ↑ Block, E. (2010). Garlic and Other Alliums: The Lore and the Science. Royal Society of Chemistry. p. 132. ISBN 978-0-85404-190-9. https://books.google.com/books?id=6AB89RHV9ucC.
- ↑ Scott, T. (1999-10-21). "What is the chemical process that causes my eyes to tear when I peel an onion?". Ask the Experts: Chemistry. Scientific American. http://www.sciam.com/article.cfm?id=what-is-the-chemical-proc.
- ↑ Kubec, R.; Cody, R. B.; Dane, A. J.; Musah, R. A.; Schraml, J.; Vattekkatte, A.; Block, E. (2010). "Applications of DART Mass Spectrometry in Allium Chemistry. (Z)-Butanethial S-Oxide and 1-Butenyl Thiosulfinates and their S-(E)-1-Butenylcysteine S-Oxide Precursor from Allium siculum". Journal of Agricultural and Food Chemistry 58 (2): 1121–1128. doi:10.1021/jf903733e. PMID 20047275.
 |


