Chemistry:Arterolane
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| Chemical and physical data | |
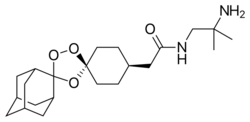
| Formula | C22H36N2O4 |
| Molar mass | 392.540 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Arterolane, also known as OZ277 or RBx 11160, is a substance that was tested for antimalarial activity[1] by Ranbaxy Laboratories.[2] It was discovered by US and European scientists who were coordinated by the Medicines for Malaria Venture (MMV).[3] Its molecular structure is uncommon for pharmacological compounds in that it has both an ozonide (trioxolane) group and an adamantane substituent.[4]
Initial results were disappointing, and in 2007 MMV withdrew support, after having invested $20M in the research;[5] Ranbaxy said at the time that it intended to continue developing the drug combination on its own.[2] Ranbaxy started a Phase II clinical trial of arterolane, in combination with piperaquine in 2009 that published in 2015.[6][7]
In 2012, Ranbaxy obtained approval to market the arterolane/piperaquine combination drug in India, under the brand name Synriam,[5] and in 2014 received approval to market it in Nigeria, Uganda, Senegal, Cameroon, Guinea, Kenya and Ivory Coast; it had already received approval in Uganda.[8]
References
- ↑ "The structure-activity relationship of the antimalarial ozonide arterolane (OZ277)". Journal of Medicinal Chemistry 53 (1): 481–91. January 2010. doi:10.1021/jm901473s. PMID 19924861.
- ↑ 2.0 2.1 "Blow to Ranbaxy drug research plans". LiveMint.com. 21 September 2007. http://www.livemint.com/2007/09/21011423/Blow-to-Ranbaxy-drugresearch.html.
- ↑ "Identification of an antimalarial synthetic trioxolane drug development candidate". Nature 430 (7002): 900–4. August 2004. doi:10.1038/nature02779. PMID 15318224. Bibcode: 2004Natur.430..900V.
- ↑ Lowe, Derek (23 November 2009). "Ozonides As Drugs: What Will They Think Of Next?". In the Pipeline. Sciencemag.org. https://www.science.org/content/blog-post/ozonides-drugs-what-will-they-think-next.
- ↑ 5.0 5.1 Rathi, Akshat (3 May 2012). "Ranbaxy launches new anti-malarial Synriam". Chemistry World. http://www.rsc.org/chemistryworld/2012/05/ranbaxy-launches-new-anti-malarial-synriam.
- ↑ "Phase II trial of dispersible fixed dose combination of arterolane (RBx 11160) maleate and piperaquine phosphate in pediatric patients with acute uncomplicated Plasmodium falciparum malaria". India Clinical trials registry. http://www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=734.
- ↑ "Efficacy and safety of fixed dose combination of arterolane maleate and piperaquine phosphate dispersible tablets in paediatric patients with acute uncomplicated Plasmodium falciparum malaria: a phase II, multicentric, open-label study". Malaria Journal 14: 469. November 2015. doi:10.1186/s12936-015-0982-y. PMID 26608469.
- ↑ Staff (16 December 2014). "Ranbaxy receives approval for malaria drug Synriam from 7 African countries". Business Standard. http://www.business-standard.com/content/b2b-pharma/ranbaxy-receives-approval-for-malaria-drug-synriam-from-7-african-countries-114121700050_1.html.
 |

