Chemistry:Calcium diglutamate
| |
| Names | |
|---|---|
| IUPAC name
Calcium bis[(2S)- 2-amino-4-carboxy-butyrate]
| |
| Other names
Calcium biglutamate, L-Glutamic acid calcium salt
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| Abbreviations | CDG, CBG |
| 11158966 | |
| ChemSpider |
|
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C10H16CaN2O8 | |
| Molar mass | 332.322 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
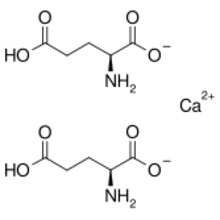
Calcium diglutamate, sometimes abbreviated CDG and also called calcium biglutamate, is a compound with formula Ca(C5H8NO4)2. It is a calcium acid salt of glutamic acid. CDG is a flavor enhancer (E number E623)—it is the calcium analog of monosodium glutamate (MSG). Because the glutamate is the actual flavor-enhancer, CDG has the same flavor-enhancing properties as MSG but without the increased sodium content.[1] Notably, only the L isomer is used in flavouring as D-glutamate does not have an umami/savoury flavour.[2][3]
As a soluble source of calcium ions, this chemical is also used as a first-aid treatment for exposure to hydrofluoric acid.[4]
Synthesis and reactions
Calcium di-glutamate can be prepared by reacting calcium carbonate with two molar equivalents of glutamic acid:[5]
CaCO3 + 2 HOOC(CH2)2CH(NH2)COOH → Ca(OOC(CH2)2CH(NH3)COO)2 + H2O + CO2↑
Concentration of the solution to a syrup under reduced pressure, followed by gradual crystallisation, affords the monohydrate.[5] Structurally, the glutamate anion is zwitterionic, with the amino group protonated (pKa = 9.47) and both carboxylic-acid groups (pKa = 2.10, 4.07) in their deprotonated carboxylate form.[6]
Calcium di-L-glutamate can be used to prepare other glutamates through metathesis with a soluble sulfate, carbonate or hydroxide salt. For example, manganese(II) di-L-glutamate can be prepared through metathesis with manganese(II) sulphate:[7]
- Ca(OOC(CH2)2CH(NH3)COO)2 + MnSO4 → Mn(OOC(CH2)2CH(NH3)COO)2 + CaSO4↓
References
- ↑ Ball, P.; Woodward, D.; Beard, T.; Shoobridge, A.; Ferrier, M. (Jun 2002). "Calcium diglutamate improves taste characteristics of lower-salt soup". European Journal of Clinical Nutrition 56 (6): 519–523. doi:10.1038/sj.ejcn.1601343. ISSN 0954-3007. PMID 12032651.
- ↑ Kawai, Misako; Sekine-Hayakawa, Yuki; Okiyama, Atsushi; Ninomiya, Yuzo (2012). "Gustatory sensation of L- and D-amino acids in humans". Amino Acids 43 (6): 2349–2358. doi:10.1007/s00726-012-1315-x. ISSN 0939-4451.
- ↑ Schiffman, S; Sennewald, K; Gagnon, J (1981). "Comparison of taste qualities and thresholds of D- and L-amino acids". Physiology & Behavior 27 (1): 51–59. doi:10.1016/0031-9384(81)90298-5.
- ↑ "First Aid for Chemical and Cleanroom Laboratories". http://www.ee.byu.edu/cleanroom/first_aid.phtml. Retrieved 2009-06-10.
- ↑ 5.0 5.1 Sakata, Yoshiki; Horikawa, Toshiyuki; Takenouchi, Kuniharu (1963). "Alkaline Earth Salts of Glutamic Acid and Optical Resolution of their Racemic Modifications". Agricultural and Biological Chemistry 27 (7): 518–525. doi:10.1080/00021369.1963.10858140. ISSN 0002-1369.
- ↑ Einspahr, H.; Bugg, C. E. (1979-02-15). "Calcium binding to α-amino acids: the crystal structure of calcium di-L-glutamate tetrahydrate". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 35 (2): 316–321. doi:10.1107/S0567740879003435.
- ↑ Devereux, Michael; Jackman, Maura; McCann, Malachy; Casey, Michael (1998). "Preparation and catalase-type activity of manganese(II) amino acid complexes". Polyhedron 17 (1): 153–158. doi:10.1016/S0277-5387(97)00211-8.
 |

