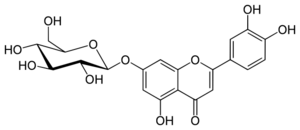
Chemistry:Cynaroside
| |
| Names | |
|---|---|
| IUPAC name
7-(β-D-Glucopyranosyloxy)-3′,4′,5-trihydroxyflavone
| |
| Systematic IUPAC name
2-(3,4-Dihydroxyphenyl)-5-hydroxy-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4H-1-benzopyran-4-one | |
| Other names
Glucoluteolin
Luteoloside Cinaroside 7-Glucoluteolin 7-Glucosylluteolin Luteolin 7-glucoside Luteolin-7-glucoside Luteolin 7-O-glucoside Luteolin-7-O-glucoside | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H20O11 | |
| Molar mass | 448.37 g/mol |
| Appearance | Yellow amorphous powder |
| Melting point | 266 to 268 °C (511 to 514 °F; 539 to 541 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cynaroside (also known as luteoloside) is a flavone, a flavonoid-like chemical compound. It is a 7-O-glucoside of luteolin.
Natural occurrences
It can be found in Ferula varia and F. foetida[1] in Campanula persicifolia and C. rotundifolia,[2] in the bamboo Phyllostachys nigra,[3] and in Teucrium gnaphalodes.[4]
- In food
It can be found in dandelion (the highest concentration in the flowers,[5] but also in the roots) and in Cynara scolymus (artichoke).[6]
Metabolism
Flavone 7-O-beta-glucosyltransferase adds a glucose to luteolin.
A cynaroside 7-O-glucosidase has been identified in the artichoke.[6]
Spectral data
| UV-Vis[7] | |
|---|---|
| Lambda-max | UV : 348, 260 nm |
| Extinction coefficient | (log ε): 4.11, 4.23 |
| IR | |
| Major absorption bands | ? cm−1 |
| NMR | |
| Proton NMR | 1H-NMR (500 MHz, CD3COCD3 + D2O): δ 3.42 (1H, t, J = 9.0 Hz, H-4′), 3.49 |
| Carbon-13 NMR | 13C-NMR (125 MHz, CD3COCD3 + D2O): δ 61.7 (C-6″), 70.3 (C-4″), 73.8 (C-2″), |
| Other NMR data | |
| MS | |
| Masses of main fragments |
ESI-MS [M+H]+ m/z 449.1 |
References
- ↑ Yuldashev, M. P. (1997). "Cynaroside content of the plants Ferula varia and F. Foetida". Chemistry of Natural Compounds 33 (5): 597–8. doi:10.1007/BF02254816.
- ↑ Teslov, L. S.; Teslov, S. V. (1972). "Cynaroside and luteolin from Campanula persicifolia and C. Rotundifolia". Chemistry of Natural Compounds 8: 117. doi:10.1007/BF00564462.
- ↑ Hu, Chun; Zhang, Ying; Kitts, David D. (2000). "Evaluation of Antioxidant and Prooxidant Activities of Bamboo Phyllostachys nigra Var. Henonis Leaf Extract in Vitro". Journal of Agricultural and Food Chemistry 48 (8): 3170–6. doi:10.1021/jf0001637. PMID 10956087.
- ↑ Flavonoid Aglycones and Glycosides from Teucrium gnaphalodes. F. A. T. Barberán, M. I. Gil, F. Tomás, F. Ferreres and A. Arques, J. Nat. Prod., 1985, 48 (5), pages 859–860, doi:10.1021/np50041a040
- ↑ "Is the Healthiest Part of Dandelion Its Flower?". 17 April 2014. https://wildfoodism.com/2014/04/17/is-the-healthiest-part-of-dandelion-its-flower/.
- ↑ 6.0 6.1 Nüβlein, B; Kreis, W (2005). "Purification and Characterization of a Cynaroside 7-O-β-D-Glucosidase from Cynarae scolymi folium". Acta Horticulturae 681 (681): 413–20. doi:10.17660/ActaHortic.2005.681.58. http://www.actahort.org/books/681/681_58.htm.
- ↑ Lin, Yi-Pei; Chen, Tai-Yuan; Tseng, Hsiang-Wen; Lee, Mei-Hsien; Chen, Shui-Tein (2009). "Neural cell protective compounds isolated from Phoenix hanceana var. Formosana". Phytochemistry 70 (9): 1173–81. doi:10.1016/j.phytochem.2009.06.006. PMID 19628235.
 |
