Chemistry:Fosaprepitant
From HandWiki
Short description: Chemical compound
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Emend, Ivemend |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a604003 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Protein binding | >95% (aprepitant) |
| Metabolism | To aprepitant |
| Elimination half-life | 9 to 13 hours (aprepitant) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
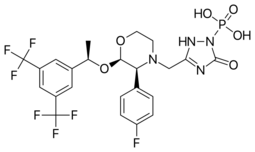
| Formula | C23H22F7N4O6P |
| Molar mass | 614.414 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fosaprepitant, sold under the brand names Emend (US) and Ivemend (EU) among others, is an antiemetic medication,[6] administered intravenously. It is a prodrug of aprepitant.
Fosaprepitant was developed by Merck & Co. and was approved for medical use in the United States,[7] and in the European Union in January 2008.[5]
References
- ↑ "Prescribing medicines in pregnancy database". 21 June 2022. https://www.tga.gov.au/products/medicines/find-information-about-medicine/prescribing-medicines-pregnancy-database.
- ↑ "Emend IV fosaprepitant 150mg (as fosaprepitant dimeglumine) powder for injection vial (167061)". 27 May 2022. https://www.tga.gov.au/resources/artg/167061.
- ↑ "Fosaprepitant MSN (Accelagen Pty Ltd)". 11 November 2022. https://www.tga.gov.au/resources/prescription-medicines-registrations/fosaprepitant-msn-accelagen-pty-ltd.
- ↑ "Emend- fosaprepitant dimeglumine injection, powder, lyophilized, for solution". 2 May 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8d66803a-6811-4c29-9c57-e16acfa87f21.
- ↑ 5.0 5.1 "Ivemend EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/ivemend.
- ↑ "Fosaprepitant Dimeglumine: A Review in the Prevention of Nausea and Vomiting Associated with Chemotherapy". Drugs 76 (14): 1365–72. September 2016. doi:10.1007/s40265-016-0627-7. PMID 27510503.
- ↑ "Drugs.com, FDA Approves Emend (fosaprepitant dimeglumine) for Injection, Merck's New Intravenous Therapy, for Use in Combination with Other Antiemetics for Prevention of Nausea and Vomiting Caused by Chemotherapy". https://www.drugs.com/newdrugs/fda-approves-emend-fosaprepitant-dimeglumine-merck-s-new-intravenous-therapy-combination-other-833.html.
 |
