Chemistry:Dimenhydrinate
 | |
| Combination of | |
|---|---|
| Diphenhydramine | Antihistamine, sedative |
| 8-chlorotheophylline | Stimulant |
| Clinical data | |
| Trade names | Dramamine, Draminate, Gravol, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607046 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth, rectal, intravascular, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Liver |
| Elimination half-life | 5.5 hours[1] (diphenhydramine component) |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| | |
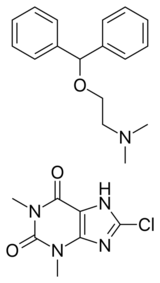
Dimenhydrinate, sold under the brand name Dramamine, among others, is an over-the-counter medication used to treat motion sickness and nausea. Dimenhydrinate is a theoclate salt composed of diphenhydramine (an ethanolamine derivative) and 8-chlorotheophylline (a chlorinated theophylline derivative) in a 1:1 ratio.[2]
Dimenhydrinate was introduced to the market by G.D. Searle in 1949.[3][4]
Medical uses
Dimenhydrinate is an over-the-counter (OTC) antihistamine indicated for the prevention and relief of nausea and vomiting from a number of causes, including motion-sickness and post-operative nausea.[2]
Side effects
Common side effects may include:[5]
- Drowsiness
- Dry mouth, nose, or throat
- Constipation
- Blurred vision
- Feeling restless or excited (especially in children)
Continuous and/or cumulative use of anticholinergic medications, including first-generation antihistamines, is associated with higher risk of cognitive decline and dementia in older people.[6][7]
Pharmacology
Diphenhydramine is the primary constituent of dimenhydrinate and dictates the primary effect. The main differences relative to pure diphenhydramine are a lower potency due to being combined with 8-chlorotheophylline (by weight, dimenhydrinate is between 53% and 55.5% diphenhydramine)[8] and the fact that the stimulant properties of 8-chlorotheophylline help reduce the side effect of drowsiness brought on by diphenhydramine. Diphenhydramine is itself an H1 receptor antagonist that demonstrates anticholinergic activity.[9]
Pharmacokinetics
The diphenhydramine component requires about 2 hours to reach peak concentration after either oral or sublingual administration of diphenhydrinate, and has a half-life of 5 – 6 hours in healthy adults.[1]
Recreational use
Dimenhydrinate is recreationally used as a deliriant.[10][11][12] Slang terms for Dramamine used this way include "drama", "dime", "dime tabs", "D-Q", "substance D", "d-house", and "drams".[13] Abusing Dramamine is sometimes referred to as Dramatizing or "going a dime a dozen", a reference to the amount of Dramamine tablets generally necessary for a trip.[14]
Many users report a side-effect profile consistent with tropane alkaloid (e.g. atropine) poisoning as both show antagonism of muscarinic acetylcholine receptors in both the central and autonomic nervous system, which inhibits various signal transduction pathways.[11]
Other CNS effects occur within the limbic system and hippocampus, causing confusion and temporary amnesia due to decreased acetylcholine signaling. Toxicology also manifests in the autonomic nervous system, primarily at the neuromuscular junction, resulting in ataxia and extrapyramidal side effects and the feeling of heaviness in the legs, and at sympathetic post-ganglionic junctions, causing urinary retention, pupil dilation, tachycardia, irregular urination, and dry red skin caused by decreased exocrine gland secretions, and mucous membranes. Considerable overdosage can lead to myocardial infarction (heart attack), serious ventricular arrhythmias, coma, and death.[15] Such a side effect profile is thought to give ethanolamine-class antihistamines a relatively low abuse liability.[citation needed] An antidote that can be used for dimenhydrinate poisoning is physostigmine.[16]
History
Dimenhydrinate (then known as Compound 1694) was being tested as a potential treatment for hay fever and hives at Johns Hopkins Hospital in 1947 by allergists Dr. Leslie Gay and Dr. Paul Carliner. Among those who received the drug was a pregnant woman who had suffered from motion sickness her entire life. She remained symptom-free if she took dimenhydrinate a few minutes before boarding a trolley, whereas the placebo was ineffective. To confirm these findings, the following year, G.D. Searle & Co. conducted a trial in which dimenhydrinate or placebo was given to U.S. troops crossing the Atlantic during "a rough passage" in a converted freight ship, the General Ballou, for 10 days as a rescue therapy for sea sickness. The findings were positive, as were the findings of a second trial of mostly women on the ship's return voyage. Gay and Carliner announced their discovery at a meeting of the Johns Hopkins Medical Society on February 14, 1949, as well as in the Bulletin of The Johns Hopkins Hospital. The New York Times , the Baltimore Sun, and other national newspapers covered the discovery, and Dramamine was made available in drugstores later that year.[3][4][17]
Brand names
Dimenhydrinate is marketed under many brand names: in the U.S., Mexico, Turkey, Serbia, and Thailand as Dramamine; in Ukraine as Driminate; in Canada, Costa Rica, and India as Gravol; in Iceland as Gravamin; in Russia and Croatia as Dramina; in South Africa and Germany as Vomex; in Australia and Austria as Vertirosan; in Brazil as Dramin; in Colombia as Mareol; in Ecuador as Anautin; in Hungary as Daedalon; in Indonesia as Antimo; in Italy as Xamamina or Valontan; in Peru as Gravicoll; in Poland and Slovakia as Aviomarin;[18] in Portugal as Viabom, Vomidrine, and Enjomin; in Spain as Biodramina; in Israel as Travamin; and in Pakistan as Gravinate.[19]
Popular culture
Modest Mouse produced a song titled "Dramamine" on their 1996 debut album This Is a Long Drive for Someone with Nothing to Think About. The song uses side effects of the drug as a metaphor for the deteriorating state of a personal relationship.[20]
References
- ↑ 1.0 1.1 "Diphenhydramine kinetics following intravenous, oral, and sublingual dimenhydrinate administration". Biopharmaceutics & Drug Disposition 11 (3): 185–189. April 1990. doi:10.1002/bdd.2510110302. PMID 2328304.
- ↑ 2.0 2.1 "34 - Pharmacology of Postoperative Nausea and Vomiting". Pharmacology and Physiology for Anesthesia (Second ed.). Elsevier Inc.. 2019. pp. 671–692. doi:10.1016/B978-0-323-48110-6.00034-X. ISBN 978-0-323-48110-6. https://www.sciencedirect.com/science/article/pii/B978032348110600034X.
- ↑ 3.0 3.1 "New Dramamine Ads Take Aim at Summer Vacationers". 21 June 2012. https://www.nytimes.com/2012/06/21/business/media/new-dramamine-ads-take-aim-at-summer-vacationers.html.
- ↑ 4.0 4.1 "Hopkins History Moments: Neil A. Grauer explains how Hopkins expertise helped prevent seasickness". 12 February 2019. https://www.hopkinsmedicine.org/news/articles/hopkins-history-moments-3.
- ↑ "Dimenhydrinate". https://www.drugs.com/mtm/dimenhydrinate.html#side-effects.
- ↑ "Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study". JAMA Internal Medicine 175 (3): 401–407. March 2015. doi:10.1001/jamainternmed.2014.7663. PMID 25621434.
- ↑ "Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study". Archives of Internal Medicine 169 (14): 1317–1324. July 2009. doi:10.1001/archinternmed.2009.229. PMID 19636034.
- ↑ "Dimenhydrinate injection, solution". U.S. National Library of Medicine. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=bc71539e-1a33-4709-8a24-c2894e8dbc1c.}}
- ↑ "Abuse and Misuse Potential of Dimenhydrinate: A Review of the Clinical Evidence [Internet]". CADTH Rapid Response Reports.. 2015. PMID 26985532.
- ↑ "The Health Risks of Abusing Motion sickness pills". http://www.narconon.org/drug-abuse/prescription/motion-sickness-pills.html.
- ↑ 11.0 11.1 "Abuse of prescription and over-the-counter medications". Journal of the American Board of Family Medicine 21 (1): 45–54. 2008. doi:10.3122/jabfm.2008.01.070071. PMID 18178702.
- ↑ "Dimenhydrinate abuse among adolescents". Canadian Journal of Psychiatry 38 (2): 113–116. March 1993. doi:10.1177/070674379303800208. PMID 8467436.
- ↑ "The Dangers of Dimenhydrinate Abuse". 6 October 2009. http://www.brighthub.com/science/medical/articles/51711.aspx.
- ↑ "Dramamine". Budderbongs forums. https://budderbongs.com/.
- ↑ "Are Teens Abusing Motion Sickness Pills? - Muir Wood Adolescent and Family Services" (in en-US). http://www.muirwoodteen.com/over-the-counter-drug-abuse/motion-sickness-pills/.
- ↑ "Diphenhydramine and dimenhydrinate poisoning: an evidence-based consensus guideline for out-of-hospital management". Clinical Toxicology 44 (3): 205–223. 19 January 2006. doi:10.1080/15563650600585920. PMID 16749537.
- ↑ "A History of Drug Discovery for Treatment of Nausea and Vomiting and the Implications for Future Research". Frontiers in Pharmacology 9: 913. 2018. doi:10.3389/fphar.2018.00913. PMID 30233361.
- ↑ "Aviomarin, tabletki, 50 mg, 5 szt" (in pl). http://www.doz.pl/apteka/p5336-Aviomarin_tabletki_50_mg_5_szt.
- ↑ "Gravinate [Dimenhydrinate"]. Karachi Pakistan: The Searle Company. http://www.searlecompany.com/gravinate.html.
- ↑ "Modest Mouse: 'This is a long drive...'". Portland: Glacial Pace Recordings. https://shop.glacialpace.com/collections/music/products/this-is-a-long-drive-for-someone-with-nothing-to-think-about.
External links
 |

