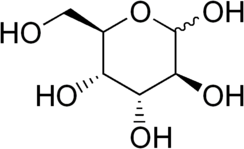
Chemistry:Altrose
From HandWiki

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Altrose | |
| Systematic IUPAC name
(2S,3R,4R)-2,3,4,5,6-Pentahydroxyhexanal | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider | |
| KEGG |
|
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C6H12O6 | |
| Molar mass | 180.156 g·mol−1 |
| Melting point | 103 to 105 °C (217 to 221 °F; 376 to 378 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Altrose is an aldohexose sugar. D-Altrose is an unnatural monosaccharide. It is soluble in water and practically insoluble in methanol. However, L-altrose has been isolated from strains of the bacterium Butyrivibrio fibrisolvens.[1]
Altrose is a C-3 epimer of mannose. The ring conformation of α-altropyranoside is flexible compared to most other aldohexopyranosides, with idose as exception. In solution different derivatives of altrose have been shown to occupy both 4C1, OS2 and 1C4-conformations.[2]
References
- ↑ "Microbial production of L-altrose" US patent 4966845, issued 1990-10-30, assigned to Government of the United States of America, Secretary of Agriculture
- ↑ Immel, Stefan; Fujita, Kahee; Lichtenthaler, Frieder W. (1999). "Solution Geometries and Lipophilicity Patterns ofα-Cycloaltrin". Chemistry - A European Journal 5 (11): 3185–3192. doi:10.1002/(SICI)1521-3765(19991105)5:11<3185::AID-CHEM3185>3.0.CO;2-W. ISSN 0947-6539.
 |
