Chemistry:Rhamnose
| |
| Names | |
|---|---|
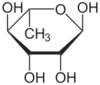
| IUPAC name
6-Deoxy-L-mannopyranose
| |
| Systematic IUPAC name
(2R,3R,4R,5R,6S)-6-Methyloxane-2,3,4,5-tetrol | |
| Other names
Isodulcit
α-L-Rhamnose L-Rhamnose L-Mannomethylose α-L-Rha α-L-Rhamnoside α-L-Mannomethylose 6-Deoxy-L-mannose L-Rhamnopyranose | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H12O5 | |
| Molar mass | 164.157 g·mol−1 |
| Density | 1.41 g/mL |
| Melting point | 91 to 93 °C (196 to 199 °F; 364 to 366 K) (monohydrate) |
| -99.20·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rhamnose (Rha, Rham) is a naturally occurring deoxy sugar. It can be classified as either a methyl-pentose or a 6-deoxy-hexose. Rhamnose predominantly occurs in nature in its L-form as L-rhamnose (6-deoxy-L-mannose). This is unusual, since most of the naturally occurring sugars are in D-form. Exceptions are the methyl pentoses L-fucose and L-rhamnose and the pentose L-arabinose. However, examples of naturally-occurring D-rhamnose include some species of bacteria, such as Pseudomonas aeruginosa and Helicobacter pylori.[2]
Rhamnose can be isolated from buckthorn (Rhamnus), poison sumac, and plants in the genus Uncaria. Rhamnose is also produced by microalgae belonging to class Bacillariophyceae (diatoms).[3]
Rhamnose is commonly bound to other sugars in nature. It is a common glycone component of glycosides from many plants. Rhamnose is also a component of the outer cell membrane of acid-fast bacteria in the Mycobacterium genus, which includes the organism that causes tuberculosis.[4] Natural antibodies against L-rhamnose are present in human serum,[5] and the majority of people seem to possess IgM, IgG or both of these types of immunoglobulins capable of binding this glycan.[6]
An interesting particularity of rhamnose is the absence of formaldehyde production when reacted with periodates in the vicinal diol cleavage reaction, that makes it very useful to remove excess periodate in glycerol or other vicinal diol analysis, that would otherwise give colored blank issues.[7]
See also
- Galactose binding lectin domain, despite the name, often binds rhamnose
- Alpha-L-rhamnosidase
Disaccharides:
- Rutinose, rhamnose-glucose
- Neohesperidose, rhamnose-glucose
- Robinose, rhamnose-galactose
Polysaccharides:
- Gellan gum -glucose-glucuronic acid-glucose-rhamnose-
- Welan gum
Glycosides:
References
- ↑ Merck Index, 11th Edition, 8171.
- ↑ "Biosynthesis of the Pseudomonas aeruginosa common polysaccharide antigen by D‐Rhamnosyltransferases WbpX and WbpY". Glycoconjugate Journal. 2022. doi:10.1007/s10719-022-10040-4. PMID 35166992.
- ↑ Brown, M. R. (1991). "The amino-acid and sugar composition of 16 species of microalgae used in mariculture". Journal of Experimental Marine Biology and Ecology 145: 79. doi:10.1016/0022-0981(91)90007-J.
- ↑ Golan, David E., ed (2005). "Chapter 35 - Pharmacology of the Bacterial Cell Wall". Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Armen H. Tashjian Jr., Ehrin J. Armstrong, Joshua N. Galanter, April Wang Armstrong, Ramy A. Arnaout, Harris S. Rose. Lippincott Williams and Wilkins. p. 569. ISBN 0-7817-4678-7.
- ↑ "Profiling Human Serum Antibodies with a Carbohydrate Antigen Microarray". J. Proteome Res. 8 (9): 4301–10. 2009. doi:10.1021/pr900515y. PMID 19624168.
- ↑ "Detection of human IgM and IgG antibodies by means of galactofuranose-coated and rhamnose-coated gold nanoparticles". Matters. 2019. https://sciencematters.io/articles/201908000004
- ↑ Ashworth, M. R. F., ed (1979). "Chapter 3". Analytical methods for glycerol. Academic Press.
Further reading
- Watanabe, K; Takesue, S (1975). "Use of L-rhamnose to Study Irreversible Adsorption of Bacteriophage PL-1 to a Strain of Lactobacillus casei". Journal of General Virology 28 (1): 29–35. doi:10.1099/0022-1317-28-1-29. PMID 239994.
 |
