Chemistry:Indacrinone
Indacrinone is a loop diuretic. It can be used in patients of gout with hypertension as an antihypertensive because it decreases reabsorption of uric acid,[1] while other diuretics increase it.
Chirality and biological activity
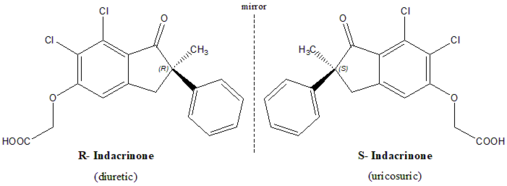
Indacrinone is a chiral drug, with one chiral center and hence exists as mirror-image twins. (R)-enantiomer, the eutomer, is diuretic whereas the mirror-image version (S)-enantiomer counteracts side effect of the eutomer. Here both the enantiomers contribute to the overall desired effect in different ways.
As indicated earlier, the (R)- enantiomer is the pharmacologically active diuretic. Like most other diuretics, the (R)-isomer possesses an undesirable side-effect of retaining uric acid. But the (S)-enantiomer, the distomer, has the property of assisting uric acid secretion (uricosuric effect), and, therefore, antagonizing the undesirable side-effects of the eutomer (uric-acid retention).[2][3] It affords a good argument for the marketing of a racemic mixture. But studies exemplify that 9:1 mixture of the two enantiomers provides optimal therapeutic value.[4]
Synthesis

The Friedel-Crafts acylation of 2,3-dichloroanisole [1984-59-4] (1) with phenylacetyl chloride [103-80-0] (2) gives 2,3-dichloro-4-phenylacetylanisole [59043-83-3] (3). A variation of the Mannich reaction is performed employing tetramethyldiaminomethane [51-80-9] (this is an aminal of dimethylamine and formaldehyde). The intermediate reaction product (5), which is not isolated, would undergo a β-Hydride elimination with concomitant loss of dimethylamine and formation of the corresponding enone, 2,3-Dichloro-4-(2-phenylacryloyl)anisole (PC10924810) (6). Acid catalyzed (H2SO4) intramolecular cyclization gives the indanone (PC10990444) (7). This is O-demethylated under acidic conditions to give 2-Phenyl-5-hydroxy-6,7-dichloro-1-indanone, PC12774089 (8). The phenol thus obtained is then alkylated on oxygen by iodoacetic acid [64-69-7] (9) affording PC20520826 (10). Alkylation with iodomethane [74-88-4] in the presence of sodium hydride completed the synthesis of indacrinone (11).
See also
References
- ↑ "Indacrinone: natriuretic and uricosuric effects of various ratios of its enantiomers in healthy men". Pharmacotherapy 4 (5): 272–7. 1984. doi:10.1002/j.1875-9114.1984.tb03374.x. PMID 6504708.
- ↑ Ariëns, Everardus J. (1986). "Stereochemistry: A source of problems in medicinal chemistry". Medicinal Research Reviews 6 (4): 451–466. doi:10.1002/med.2610060404. ISSN 0198-6325. PMID 3534485. http://dx.doi.org/10.1002/med.2610060404.
- ↑ Kannappan, Valliappan. "Indacrinone – Chiralpedia" (in en-US). https://chiralpedia.com/blog/indacrinone/.
- ↑ The impact of stereochemistry on drug development and use. Hassan Y. Aboul-Enein, Irving W. Wainer. New York: Wiley. 1997. ISBN 0-471-59644-2. OCLC 35262289.
- ↑ Castaer, J.; Chatterjee, S.S.; MK 196. Drugs Fut 1977, 2, 3, 179.
- ↑ Desolms, S. J.; Woltersdorf, O. W.; Cragoe, E. J.; Watson, L. S.; Fanelli, G. M. (1978). "(Acylaryloxy)acetic acid diuretics. 2. (2-Alkyl-2-aryl-1-oxo-5-indanyloxy)acetic acids". Journal of Medicinal Chemistry 21 (5): 437. doi:10.1021/jm00203a006.
- ↑ Edward J. Cragoe, Jr. & Otto W. WOLTERSDORF, Jr., U.S. Patent 4,096,267 (1978 to Merck and Co Inc).
- ↑ Edward H. Blaine, et al. U.S. Patent 4,510,322 (1985 to Merck and Co Inc).
- ↑ Dolling, Ulf H.; Davis, Paul; Grabowski, Edward J. J. (1984). "Efficient catalytic asymmetric alkylations. 1. Enantioselective synthesis of (+)-indacrinone via chiral phase-transfer catalysis". Journal of the American Chemical Society. 106 (2): 446–447. doi:10.1021/ja00314a045.
 |
