Chemistry:Bumetanide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Bumex, Burinex, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684051 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Almost complete (~80%) |
| Protein binding | 97% |
| Metabolism | Liver |
| Elimination half-life | ~0.8 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
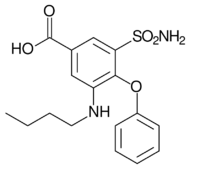
| Formula | C17H20N2O5S |
| Molar mass | 364.42 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Bumetanide, sold under the brand name Bumex among others, is a medication used to treat swelling and high blood pressure.[1] This includes swelling as a result of heart failure, liver failure, or kidney problems.[1] It may work for swelling when other medications have not.[1] For high blood pressure it is not a preferred treatment.[1] It is taken by mouth, or by injection into a vein or muscle.[1] Effects generally begin within an hour and lasts for about six hours.[1]
Common side effects include dizziness, low blood pressure, low blood potassium, muscle cramps, and kidney problems.[1] Other serious side effects may include hearing loss and low blood platelets.[1] Blood tests are recommended regularly for those on treatment.[1] Safety during pregnancy and breastfeeding is unclear.[2] Bumetanide is a loop diuretic and works by decreasing the reabsorption of sodium by the kidneys.[3][1]
Bumetanide was patented in 1968 and came into medical use in 1972.[4] It is on the World Health Organization's List of Essential Medicines.[5] It is available as a generic medication.[3] In 2020, it was the 270th most commonly prescribed medication in the United States, with more than 1 million prescriptions.[6][7]
Medical uses
It is used to treat swelling and high blood pressure.[1] This includes swelling as a result of heart failure, liver failure, or kidney problems.[1] For high blood pressure it is not a preferred treatment.[1] It is taken by mouth, or by injection into a vein or muscle.[1]
Side effects
Common side effects include dizziness, low blood pressure, low blood potassium, muscle cramps, and kidney problems.[1] Other serious side effects may include hearing loss and low blood platelets.[1] A large observational study [8] concluded that people with a sulfonamide antibiotic allergy may be allergic to sulfonamide non-antibiotics, such as bumetanide, but this is likely due to certain people being at an increased risk in general to developing allergic reactions rather than cross-reactivity between sulfonamide-containing drugs. In smaller studies, the lack of cross-reactivity between sulfonamide antibiotics and sulfonamide non-antibiotics has been demonstrated. [9][10]
Safety during pregnancy and breastfeeding is unclear.[2]
Pharmacology
Pharmacodynamics
Bumetanide is a loop diuretic and works by decreasing the reabsorption of sodium by the kidneys. The main difference between bumetanide and furosemide is in their bioavailability and potency. About 60% of furosemide is absorbed in the intestine, and there are substantial inter- and intraindividual differences in bioavailability (range 10-90%). About 80% of bumetanide is absorbed, and its absorption does not change when it is taken with food. It is said to be a more predictable diuretic, meaning that the predictable absorption is reflected in a more predictable effect.[11] Bumetanide is 40 times more potent than furosemide for people with normal renal function.[11]
Synthesis
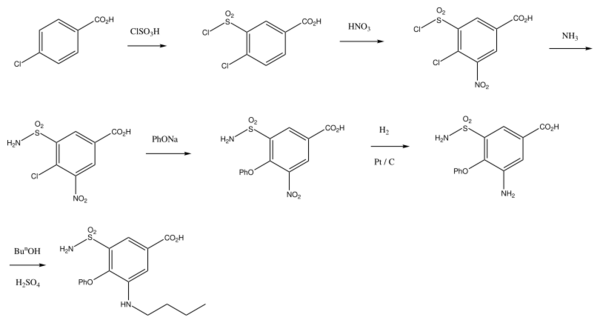
Bumetanide is synthesized from 4-chlorobenzoic acid.[12][13][14][15] In the first stage of synthesis, it undergoes sulfonylchlorination by chlorosulfonic acid, forming 4-chloro-3-chlorosulfonylbenzoic acid, which is further nitrated with nitric acid to 4-chloro-3-chlorosulfonyl-5-nitrobenzoic acid. Reacting this with ammonia gives 5-aminosulfonyl-4-chloro-3-nitrobenzoic acid, which when reacted with sodium phenolate is transformed into 5-amino-sulfonyl-3-nitro-5-phenoxybenzoic acid. Reduction of the nitro group in this product by hydrogen using a palladium on carbon catalyst gives 3-amino-5-aminosulfonyl-5-phenoxybenzoic acid. Finally, reacting this with butyl alcohol in the presence of sulfuric acid, followed by treatment with sodium hydroxide to hydrolyze the butyl ester, gives the desired bumetanide.
Society and culture
It 2008, ESPN reported that four NFL players were being suspended under the steroid policy as a result of taking bumetanide.[16]
Bumetanide was an undisclosed active ingredient in the over-the-counter weight loss supplement StarCaps, which was removed from the market after its presence was discovered by the United States Food and Drug Administration.[17]
Research
In the brain, bumetanide blocks the NKCC1 cation-chloride co-transporter, and thus decreases internal chloride concentration in neurons. In turn, this concentration change makes the action of GABA more hyperpolarizing, which may be useful for treatment of neonatal seizures, which quite often are not responsive to traditional GABA-targeted treatment, such as barbiturates. Bumetanide is therefore under evaluation as a prospective antiepileptic drug.[18]
The drug has also been studied as a treatment for autism.[19][20]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 "Bumetanide Monograph for Professionals" (in en). American Society of Health-System Pharmacists. https://www.drugs.com/monograph/bumetanide.html.
- ↑ 2.0 2.1 "Bumetanide (Bumex) Use During Pregnancy" (in en). https://www.drugs.com/pregnancy/bumetanide.html.
- ↑ 3.0 3.1 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 225–226. ISBN 9780857113382.
- ↑ (in en) Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 458. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA458.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Bumetanide - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Bumetanide.
- ↑ "Absence of cross-reactivity between sulfonamide antibiotics and sulfonamide nonantibiotics". The New England Journal of Medicine 349 (17): 1628–1635. October 2003. doi:10.1056/NEJMoa022963. PMID 14573734.
- ↑ "Sulfonamide allergies and outcomes related to use of potentially cross-reactive drugs in hospitalized patients". Pharmacotherapy 26 (4): 551–557. April 2006. doi:10.1592/phco.26.4.551. PMID 16553515.
- ↑ "Cross-reactivity among p-amino group compounds in sulfonamide fixed drug eruption: diagnostic value of patch testing". Contact Dermatitis 51 (2): 57–62. August 2004. doi:10.1111/j.0105-1873.2004.00274.x. PMID 15373844.
- ↑ 11.0 11.1 Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. 2006. pp. 749–753. ISBN 0-07-142280-3.
- ↑ Feit PW, "Arzneimittelzubereitung mit einem Gehalt an 3-Butylamino-4-phenoxy-5-sulfamyl-benzoesaeure und deren Salzen", DE patent 1964504, issued 9 July 1970, assigned to Leo Pharma Products, Ltd.
- ↑ "Aminobenzoic acid diuretics. 2. 4-Substituted-3-amino-5-sulfamylbenzoic acid derivatives". Journal of Medicinal Chemistry 14 (5): 432–9. May 1971. doi:10.1021/jm00287a014. PMID 5117690.
- ↑ Feit PW, "Pharmaceutical composition for the treatment of oedematous conditions and hypertension", US patent 3634583, issued 11 January 1972, assigned to Leo Pharmaceutical Products Ltd AS
- ↑ Feit PW, Nielsen OB, Bruun H, Bretting CA, "Sulphonamides, compositions containing the same and methods for using the same in the treatment of hypertension or odemeas", US patent 4082851, issued 4 April 1978, assigned to Leo Pharmaceutical Products Ltd AS
- ↑ "McAllister, Smith, Grant, Texans' Pittman among players testing positive". ESPN. October 26, 2008. http://www.espn.com/nfl/news/story?id=3661845.
- ↑ Food and Drug Administration Office of Criminal Investigations (March 26, 2014). "Pills Sold Throughout the United States Contained an Undisclosed Prescription Drug Banned By the National Football League". U.S. Department of Justice Press Release. United States Food and Drug Administration. https://www.fda.gov/iceci/criminalinvestigations/ucm391757.htm.
- ↑ "Cation-chloride cotransporters NKCC1 and KCC2 as potential targets for novel antiepileptic and antiepileptogenic treatments". Neuropharmacology 69: 62–74. June 2013. doi:10.1016/j.neuropharm.2012.05.045. PMID 22705273.
- ↑ "Bumetanide for Core Symptoms of Autism Spectrum Disorder (BAMBI): A Single Center, Double-Blinded, Participant-Randomized, Placebo-Controlled, Phase-2 Superiority Trial". Journal of the American Academy of Child and Adolescent Psychiatry 60 (7): 865–876. July 2020. doi:10.1016/j.jaac.2020.07.888. PMID 32730977. "Recent trials have indicated positive effects of bumetanide in autism spectrum disorder (ASD).".
- ↑ "Symptom improvement in children with autism spectrum disorder following bumetanide administration is associated with decreased GABA/glutamate ratios". Translational Psychiatry 10 (1): 9. January 2020. doi:10.1038/s41398-020-0692-2. PMID 32066666.
External links
- "Bumetanide". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/bumetanide.
title =
- "Repurposed drug boosts the positive effect of microglia to improve cognitive performance following brain trauma". https://www.helsinki.fi/en/hilife-helsinki-institute-life-science/news/repurposed-drug-boosts-positive-effect-microglia-improve-cognitive-performance-following-brain-trauma.
 |