Chemistry:Cyclothiazide
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C14H16ClN3O4S2 |
| Molar mass | 389.87 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Cyclothiazide (Anhydron, Acquirel, Doburil, Fluidil, Renazide, Tensodiural, Valmiran), sometimes abbreviated CTZ, is a benzothiadiazide (thiazide) diuretic and antihypertensive that was originally introduced in the United States in 1963 by Eli Lilly and was subsequently also marketed in Europe and Japan .[1][2] Related drugs include diazoxide, hydrochlorothiazide, and chlorothiazide.[3]
In 1993, it was discovered that cyclothiazide is a positive allosteric modulator of the AMPA and kainate receptors, capable of reducing or essentially eliminating rapid desensitization of the former receptor, and potentiating AMPA-mediated glutamate currents by as much as 18-fold at the highest concentration tested (100 μM).[3][4][5][6] Additionally, in 2003, cyclothiazide was also found to act as a GABAA receptor negative allosteric modulator, potently inhibiting GABAA-mediated currents.[7] In animals it is a powerful convulsant, robustly enhancing epileptiform activity and inducing seizures, but without producing any apparent neuronal death.[8][9]
Cyclothiazide has been found to act as a non-competitive antagonist of the mGluR1.[10] It is selective for mGluR1 over other metabotropic glutamate receptors.[10]
Synthesis
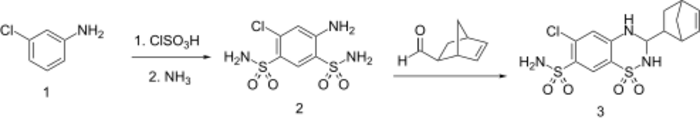
See also
References
- ↑ Swiss Pharmaceutical Society (2000). Index Nominum 2000: International Drug Directory (Book with CD-ROM). Boca Raton: Medpharm Scientific Publishers. pp. 1932. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&q=Cyclothiazide&pg=PA287.
- ↑ Pharmaceutical manufacturing encyclopedia. Park Ridge, N.J., U.S.A: Noyes Publications. 1988. pp. 1756. ISBN 978-0-8155-1144-1. https://books.google.com/books?id=X2EyLsG4bcUC&q=Cyclothiazide&pg=PA418.
- ↑ 3.0 3.1 Direct and allosteric control of glutamate receptors. Boca Raton: CRC Press. 1994. pp. 174. ISBN 978-0-8493-8307-6. https://books.google.com/books?id=Fp9RLJTt7NkC&q=Cyclothiazide&pg=PA65.
- ↑ "Benzothiadiazides inhibit rapid glutamate receptor desensitization and enhance glutamatergic synaptic currents". The Journal of Neuroscience 13 (9): 3904–3915. September 1993. doi:10.1523/JNEUROSCI.13-09-03904.1993. PMID 8103555.
- ↑ "Modulation of AMPA/kainate receptors by analogues of diazoxide and cyclothiazide in thin slices of rat hippocampus". Receptors & Channels 1 (4): 267–278. 1993. PMID 7915948.
- ↑ "Excitatory Amino Acid Neurotransmission". Anxiety and anxiolytic drugs. Berlin: Springer. 2005. pp. 566. ISBN 978-3-540-22568-3. https://books.google.com/books?id=RDP4_sBnsl4C&q=Cyclothiazide&pg=PA256.
- ↑ "Cyclothiazide potently inhibits gamma-aminobutyric acid type A receptors in addition to enhancing glutamate responses". Proceedings of the National Academy of Sciences of the United States of America 100 (22): 13025–13029. October 2003. doi:10.1073/pnas.2133370100. PMID 14534329. Bibcode: 2003PNAS..10013025D.
- ↑ "Cyclothiazide induces robust epileptiform activity in rat hippocampal neurons both in vitro and in vivo". The Journal of Physiology 571 (Pt 3): 605–618. March 2006. doi:10.1113/jphysiol.2005.103812. PMID 16423850.
- ↑ "Cyclothiazide induces seizure behavior in freely moving rats". Brain Research 1355: 207–213. October 2010. doi:10.1016/j.brainres.2010.07.088. PMID 20678492.
- ↑ 10.0 10.1 "Cyclothiazide selectively inhibits mGluR1 receptors interacting with a common allosteric site for non-competitive antagonists". Neuropharmacology 52 (3): 744–754. March 2007. doi:10.1016/j.neuropharm.2006.09.018. PMID 17095021.
- ↑ "Diuretics. V. 3,4-Dihydro-1,2,4-benzothiadiazine 1,1-Dioxides". The Journal of Organic Chemistry 26 (8): 2814–2818. 1961. doi:10.1021/jo01066a046.
- ↑ Müller E, Hasspacher K, US patent 3275625, issued 1966, assigned to Boehringer Ingelheim
 |
