Chemistry:Lactofen
| |
| Names | |
|---|---|
| IUPAC name
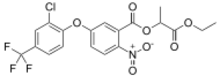
Ethyl O-[5-(2-chloro-α,α,α-trifluoro-p-tolyloxy)-2-nitrobenzoyl]-DL-lactate
| |
| Other names
2-Ethoxy-1-methyl-2-oxoethyl 5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C19H15ClF3NO7 | |
| Molar mass | 461.77 g·mol−1 |
| Appearance | White crystalline solid |
| Melting point | 43.9 to 45.5 °C (111.0 to 113.9 °F; 317.0 to 318.6 K) |
| 0.1 mg/L @ 20 degrees C | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lactofen is a complex ester of acifluorfen and is a nitrophenyl ether selective herbicide[2][3] and fungicide.[4][5][6] It is used in postemergence applications to certain crops which are resistant to its action.[2] The name "Lactofen" is approved by the American National Standards Institute and the Weed Science Society of America, and is also approved in China (乳氟禾草灵).
Lactofen is applied as a foliar spray and is commonly used to control broadleaved weeds in soybean, cereals, potatoes and peanuts. It may be combined with oil or fertilizer adjuvants and surfactants. Some formulations include solvents such as xylenes and cumene.[1] It is also used as a fungicide for Sclerotinia white molds on soybean.[4][5][6]
Lactofen is available in solid form or as an emulsifiable concentrate under the trade name COBRA.[1]
Toxicology
Lactofen is slightly to non-toxic to humans when ingested or inhaled. It can cause skin irritation including reddening, swelling and possibly corrosive burns. It is a severe eye irritant and can cause permanent damage to eyes when there is sufficient exposure.[7]
It was found to be practically non-toxic to the species of bird that were studied.[8] Toxicity to fish and other aquatic organisms varied and it was eliminated in fish within fourteen days. It is of low toxicity to bees.[9]
Lactofen has a very low solubility in water and is not expected to contaminate surface waters. It binds tightly to soil and is then broken down in between one and seven days.[8][10]
References
- ↑ 1.0 1.1 1.2 EXTOXNET: Extension Toxicology Network
- ↑ 2.0 2.1 Herbicide Mode-of-Action Summary
- ↑ SDSU Extension. 2019 South Dakota Pest Management Guide - Soybeans - A guide to managing weeds, insects, and diseases. http://extension.sdstate.edu/sites/default/files/2019-01/P-00010_0.pdf.
- ↑ 4.0 4.1 "White Mold in Soybeans - Crops". http://www.ag.ndsu.edu/crops/soybean-articles/white-mold-in-soybeans.
- ↑ 5.0 5.1 "Applying foliar fungicides for control of white mold in soybeans". http://www.canr.msu.edu/news/applying_foliar_fungicides_for_control_of_white_mold_in_soybeans.
- ↑ 6.0 6.1 Kichler, Jeremy (2021-07-19). "Questions of the Week!! 7/19/21 - Colquitt County Ag Report". http://site.extension.uga.edu/colquittag/2021/07/questions-of-the-week-7-19-21/.
- ↑ Valent USA. 1993. Material Safety Data Sheet for Valent Cobra Herbicide. Valent USA Corporation. Walnut Creek, CA.
- ↑ 8.0 8.1 Herbicide Handbook of the Weed Science Society of America. 1989. Sixth edition. Champaign, IL
- ↑ US Environmental Protection Agency. 1995. File: Lactofen, Integrated Risk Information System (IRIS). National Library of Medicine "Toxline" Database, 4/95.
- ↑ US Environmental Protection Agency. 1987. Fact Sheet Number 128: Lactofen. Washington, DC.
External links
- Lactofen in the Pesticide Properties DataBase (PPDB)
 |
