Chemistry:Latanoprostene bunod
From HandWiki
Short description: Chemical compound
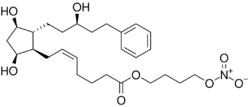 | |
| Clinical data | |
|---|---|
| Trade names | Vyzulta |
| Other names | BOL-303259-X |
| AHFS/Drugs.com | Multum Consumer Information |
| License data | |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C27H41NO8 |
| Molar mass | 507.624 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Latanoprostene bunod (trade name Vyzulta) is an ophthalmic drug approved in the United States in 2017 for the reduction of intraocular pressure in patients with open-angle glaucoma or ocular hypertension.[3][4] It targets the trabecular meshwork directly.[4]
References
- ↑ "Search Page - Drug and Health Product Register". 23 October 2014. https://hpr-rps.hres.ca/reg-content/regulatory-decision-summary-detail.php?lang=en&linkID=RDS00499.
- ↑ "Vyzulta- latanoprostene bunod solution/ drops". 1 May 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=60ce7900-a677-4fc2-88d0-1603baa9dbd4.
- ↑ "FDA Approves Vyzulta (latanoprostene bunod) Ophthalmic Solution for Open-Angle Glaucoma, Ocular Hypertension" (Press release). Valeant Pharmaceuticals International, Inc.
- ↑ 4.0 4.1 "Latanoprostene Bunod Ophthalmic Solution 0.024%: A Review in Open-Angle Glaucoma and Ocular Hypertension". Drugs 78 (7): 773–780. May 2018. doi:10.1007/s40265-018-0914-6. PMID 29761382.
 |

