Chemistry:Mepivacaine
From HandWiki
Short description: Local anaesthetic
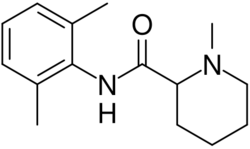 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a603026 |
| Pregnancy category |
|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C15H22N2O |
| Molar mass | 246.354 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Mepivacaine /mɛˈpɪvəkeɪn/ is a local anesthetic[1] of the amide type. Mepivacaine has a reasonably rapid onset (less rapid than that of procaine) and medium duration of action (longer than that of procaine)[2][3] and is marketed under various trade names including Carbocaine and Polocaine.
Mepivacaine became available in the United States in the 1960s.
Mepivacaine is used in any infiltration and local anesthesia.
It is supplied as the hydrochloride salt of the racemate,[4] which consists of R(-)-mepivacaine and S(+)-mepivacaine in equal proportions. These two enantiomers have markedly different pharmacokinetic properties.[4]
Mepivacaine was originally synthesized in Sweden at the laboratory of Bofors Nobelkrut in 1956.[5]
References
- ↑ "Evaluation of lidocaine and mepivacaine for inferior third molar surgery". Medicina oral, patología oral y cirugía bucal 12 (1): E60–4. January 2007. PMID 17195831. http://www.medicinaoral.com/medoralfree01/v12i1/medoralv12i1p60.pdf.
- ↑ "Procaine". https://go.drugbank.com/drugs/DB00721.
- ↑ "Mepivacaine". https://go.drugbank.com/drugs/DB00961.
- ↑ 4.0 4.1 "Pharmacokinetics of the enantiomers of mepivacaine after intravenous administration of the racemate in volunteers". Anesthesia & Analgesia 84 (1): 85–9. January 1997. doi:10.1097/00000539-199701000-00016. PMID 8989005.
- ↑ Castrén, J.A. (1963). "A clinical evaluation of mepivacaine (Carbocain) in ocular surgery". Acta Ophthalmologica 41 (3): 262–9. doi:10.1111/j.1755-3768.1963.tb02436.x. PMID 14047466.
External links
 |
