Chemistry:Methylsulfonylmethane
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
(Methanesulfonyl)methane | |
| Other names
methyl sulfone
methylsulfonylmethane sulfonylbismethane DMSO2 | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | MSM |
| 1737717 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 130437 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C2H6O2S | |
| Molar mass | 94.13 g·mol−1 |
| Appearance | White crystalline solid |
| Density | 1.45 g/cm3 |
| Melting point | 109 °C (228 °F; 382 K) |
| Boiling point | 248[1] °C (478 °F; 521 K) |
| Acidity (pKa) | 31 |
| Hazards[3] | |
| Safety data sheet | External MSDS |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H319 | |
| P264, P280, P305+351+338, P337+313 | |
| NFPA 704 (fire diamond) | |
| Flash point | 143 °C (289 °F; 416 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
5g/kg (oral, rat) [2] |
| Related compounds | |
Related compounds
|
DMSO dimethyl sulfide dimethyl sulfate sulfolane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
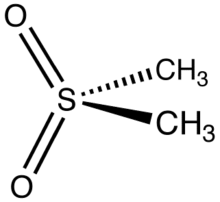
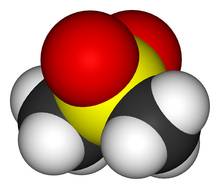
Dimethyl sulfone (DMSO2) is an organosulfur compound with the formula (CH3)2SO2. It is also known by several other names including methyl sulfone and (especially in alternative medicine) methylsulfonylmethane (MSM).[4] This colorless solid features the sulfonyl functional group and is the simplest of the sulfones. It is relatively inert chemically and is able to resist decomposition at elevated temperatures. It occurs naturally in some primitive plants, is present in small amounts in many foods and beverages, and is marketed (under the MSM name) as a dietary supplement. It is sometimes used as a cutting agent for illicitly manufactured methamphetamine.[5] It is also commonly found in the atmosphere above marine areas, where it is used as a carbon source by the airborne bacteria Afipia.[6] Oxidation of dimethyl sulfoxide produces the sulfone, both under laboratory conditions and metabolically.[7]
Use as a solvent
Because of its polarity and thermal stability, molten DMSO2 has been used industrially as a high-temperature solvent. For example, displacement of aryl chlorides by potassium fluoride has been conducted in the liquid.[8]
With a pKa of 31, the sulfone can be deprotonated with sodium amide. The conjugate base has been used as a nucleophile.
Pharmacology and toxicity
Nuclear magnetic resonance (NMR) studies have demonstrated that oral doses of MSM are absorbed into the blood and cross the blood/brain barrier.[9][10] An NMR study has also found detectable levels of MSM normally present in the blood and cerebrospinal fluid, suggesting that it derives from dietary sources, intestinal bacterial metabolism, and the body's endogenous methanethiol metabolism.[11]
Medical and dietary use
Although no medical uses for MSM have been approved, a variety of health benefits have been claimed and studied. Stanley W. Jacob reported having administered MSM to over 18,000 patients with a variety of ailments;[12] he co-authored a book promoting MSM with a variety of claims, including a utility as a natural source of "biologically active sulfur,"[13] suggesting that people are deficient in such forms of sulfur in their dietary intake. There is no Dietary Reference Intake (DRI) or Daily Value established for sulfur but notable dietary sources include cruciferous vegetables, garlic, onions, asafoetida, legumes, nuts, seeds, plant milk, animal milk and eggs (whites and yolks).[14]
The claims for the need for sulfur supplementation originate with Robert Herschler, a biochemist who patented "Dietary and pharmaceutical uses of methylsulfonylmethane and compositions comprising it" in 1982. He claimed that MSM was useful in stress, mucous-membrane inflammation, allergies and gastrointestinal conditions.[15]
Moreover, in cases involving topical therapeutics, the role of MSM as an active agent, per se, versus its having a role in promoting skin permeation (in manner, akin to its solvent relative DMSO) must be characterized/controlled.[16] The biochemical effects of supplemental methylsulfonylmethane are poorly understood. Some researchers have suggested that MSM has anti-inflammatory effects.[17] The spectrum of biological effects of dimethyl sulfoxide (DMSO) and MSM differ, but those of DMSO may be mediated, at least in part, by MSM.[18]
U.S. FDA action
In July 2007 a manufacturer of MSM submitted a notification to the U.S. FDA claiming generally recognized as safe (GRAS) status. GRAS status is for safety, and has no evaluation of efficacy. The FDA responded in February 2008 with a letter of non-objection, functionally designating OptiMSM, the branded form of MSM, as GRAS. The designation allows MSM to be added to meal supplement and meal replacement foods, fruit smoothie-type drinks, fruit-flavored thirst quencher-type beverages, and food bars such as granola bars and energy-type bars.[19]
Evidence from clinical trials
Small-scale studies of possible treatments with MSM have been conducted on both animals and humans. These studies of MSM have suggested some benefits, particularly for treatment of oxidative stress and osteoarthritis, but evidence for other uses is lacking. Natural Medicines Comprehensive Database contains a continually updated list of health-related MSM studies.[20]
Cancer
A South Korean study focussing on the role of MSM affecting growth factors associated with breast cancer identifies MSM to have multiple targets, both in vitro and in vivo, including STAT3, STAT5b, IGF-1R and VEGF, confirming the ability of MSM to suppress tumor initiation, growth and metastasis in a dose dependent manner. The expression of triple negative hormone receptors is also down regulated by MSM. In a xenograft model the mice showed inhibited tumor cell migration and suppressed tumor growth in a dose dependent manner when receiving MSM as part of their drinking water at nul, three or five percent MSM weight over volume. The authors strongly recommend MSM as a trial drug for treating breast cancers because of its multi-targeting mechanism.[21]
HDL cholesterol
A small 2021 Randomized Controlled Trial on cardiometabolic markers found a possible relationship between a daily dose of 3 g of MSM and high-density lipoprotein (HDL) levels on overweight and obese people. The MSM group demonstrated higher HDL levels after 16 weeks (51.8 ± 2.8 mg/dL) when compared to the baseline (44.9 ± 3.7 mg/dL) and also versus the placebo group (42.7 ± 2.5 mg/dL at baseline vs 48.0 ± 4.9 mg/DL at 16 weeks). Other markers (inflammation, fasting glucose, resting heart rate, etc.) showed no significant changes between baseline and 16 weeks on either the placebo or the MSM groups with the exception of C-reactive protein levels, which were higher on the placebo group than on the MSM group at baseline and continued to increase up to the 16 weeks mark while showing a very slight decrease on the MSM group. However, the authors state they cannot explain why CRP levels were higher on the placebo group nor whether MSM may play a stabilizing role on CRP. The authors also state more and larger studies are required to establish the relationship between MSM and HDL.[22]
Safety
The -1">50 of MSM is greater than 17.5 grams per kilogram of body weight. In rats, no adverse events were observed after daily doses of 2 g MSM per kg of body weight. In a 90-day follow-up study, rats received daily MSM doses of 1.5 g/kg, and no changes were observed in terms of symptoms, blood chemistry or gross pathology.[23]
Extensive research in animal models indicates MSM has a very low toxicity when administered both orally and topically.[24][25][26]
In clinical trials, several studies reported minimal or absence of side effects after 12 weeks of dosing. Reported side effects from these studies included mild gastrointestinal issues, fatigue, and headache, although they did not appear to differ from placebo.[27][28] A more recent 26-week study on large joint osteoarthritis observed no adverse events or abnormal changes in lab monitoring when taking 6 grams MSM per day.[29] MSM is considered 'Possibly Safe' at therapeutic doses, although further research is still needed to assess its safety for long-term use.[20][30]
Osteoarthritis
A review of two small randomized controlled trials of methylsulfonylmethane in osteoarthritis (OA) knee pain relief[27][31] "reported significant improvement in pain outcomes in the treatment group compared to comparator treatments; however, methodological issues and concerns over optimal dosage and treatment period were highlighted." The two trials included 168 people, of whom 52 received MSM, either 1.5 g/day or 6.0 g/day. The review authors stated: "No definitive conclusion can currently be drawn" and there is "no definitive evidence that MSM is superior to placebo in the treatment of mild to moderate osteoarthritis of the knee."[32]
Subsequent to the 2008 review there have been two more clinical trials :
- One was a double-blind, randomized, placebo controlled trial with 49 participants taking 1.125 g of MSM or placebo three times daily for 12 weeks. The results showed a significant decrease in WOMAC physical function and total WOMAC scores, as well as improvement in VAS pain scores. The effect size of MSM supplementation was slightly lower than that of NSAID use as reported in other clinical trials. The authors wrote "longer-term trials may yield additional and greater improvements."[28]
- The second used 6.0 g/day versus placebo for 26 weeks. Subjects were evaluated through the WOMAC questionnaire, SF-36 Quality of Life survey, and Global Assessments for OA symptoms from both patients and physicians. WOMAC results showed significant improvements in all areas for the MSM group. The MSM group also showed a strong trend towards changes in disease status. Careful lab monitoring of health indicators showed no side effects of MSM supplementation and no adverse events were reported.[29]
Synergism of methylsulfonylmethane and glucosamine and chondroitin
There are findings from Indian researchers that show an improvement of symptoms like pain, inflammation and swelling in patients by a combined intake of MSM and glucosamine.[31] Two other studies investigated the effects of MSM in combination with glucosamine and chondroitin (GC) on joint function. In the first study, the GC+MSM group saw significant benefits sooner than the GC group. This suggests that the addition of MSM reduces the time to benefit.[33] In another, the GC+MSM group saw significant improvements in both WOMAC and VAS pain scores when compared to both the placebo group and GC group. This suggests that the addition of MSM increases the magnitude of benefits.[34]
Skin, hair, nails
The first scientific investigation of MSM for skin health was published in 2015. The RCT used a 3g per day dose and included only 20 women. Results showed significant improvements in the number and severity of facial wrinkles, firmness, tone and texture.[35] Another study evaluated doses of 1g and 3g and showed improvements in wrinkles, firmness, and hydration at both dose levels in 20 persons.[36] The same author published a study on MSM for hair and nails from the same clinical trial. The results showed improvements in hair shine, volume, and appearance, and nail shine and appearance.[37] An Italian study evaluated the effects of a nutraceutical composed of MSM, hyaluronic acid, and L-carnosine. The results from RCT showed broad improvements in facial skin hydration and elasticity, as well as decreased sebaceous secretions.[38]
Oxidative stress and inflammation
Multiple human and animal trials indicate MSM may reduce oxidative stress and inflammation. In one small human trial, MSM has been shown to protect muscles from damage by reducing the amount of oxidative stress damage incurred through exercise.[39][40] In a second small trial the total antioxidant capacity was significantly increased after taking MSM.[41] Studies in animals indicate a hepatoprotective effect of MSM against several toxins including acetaminophen, paraquat, and carbon tetrachloride.[42][43][44][45] Animal models of experimental colitis and pulmonary hypertension indicate a protective effect as well.[46][47]
Allergies and immunity
Two studies have evaluated the effects of MSM on allergic rhinitis. A 2004 multi-centered, open-label clinical trial found that MSM reduced both upper and lower respiratory symptoms associated with seasonal allergic rhinitis (SAR), and increased energy levels. It found no significant changes in IgE levels, although the duration of the study was not likely long enough to see changes.[48] An RCT evaluated three doses of MSM and found that a 3g daily dose was most effective compared to 1g or 6g per day. Daily use at 3g decreased allergy-associated symptoms, including itchy eyes, itchy nose, watery eyes, rhinorrhea, sneezing, and nasal obstruction. The 3g dose also improved peak nasal inspiratory flow (PNIF) indicating improved breathing. The study also evaluated an acute 12g dose and found significant improvements in all symptoms except itching eyes and sneezing, but not for PNIF.[49]
MSM has been shown to improve immune function markers. RCT found that in blood samples taken after bouts of exhaustive exercise, there was a reduced response to an infectious stimulus in the placebo group, but the MSM group maintained a robust response, indicating that MSM protected against stress-induced immunosuppression. The authors postulate that MSM’s anti-inflammatory properties reduce the overstimulation of inflammatory cells during exercise, thus conserving their ability to respond to infections threats.[50] This is supported by in vitro research showing MSM inhibits over-activation of white blood cells and has an anti-apoptotic effect.[51][52]
Notes
- ↑ Gaylord Chemical Company, LLC
- ↑ "Dimethyl sulfone". https://pubchem.ncbi.nlm.nih.gov/compound/Dimethyl-sulfone#section=Toxicity.
- ↑ "Dimethyl sulfone" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/6213#section=Safety-and-Hazards.
- ↑ "Various Names for MSM". http://www.gaylordchemical.com/bulletins/Bulletin301B/Bulletin301B.pdf.
- ↑ "Information Bulletin: Crystal Methamphetamine". https://www.justice.gov/archive/ndic/pubs1/1837/1837t.htm.
- ↑ "Microbiome of the upper troposphere: species composition and prevalence, effects of tropical storms, and atmospheric implications". Proceedings of the National Academy of Sciences of the United States of America 110 (7): 2575–80. February 2013. doi:10.1073/pnas.1212089110. PMID 23359712. Bibcode: 2013PNAS..110.2575D. "This group [Afipia] is commonly found in aquatic environments and is known to use dimethyl sulfone (DMSO2) as a sole carbon source. DMSO2 represents an intermediate of the oxidation of dimethyl sulfide (DMS), which is commonly found in the marine atmosphere".(page 5 of 6, quote slightly edited).
- ↑ "Metabolic fingerprint of dimethyl sulfone (DMSO2) in microbial-mammalian co-metabolism". Journal of Proteome Research 13 (12): 5281–92. December 2014. doi:10.1021/pr500629t. PMID 25245235. http://www.escholarship.org/uc/item/7w78k2pt.
- ↑ Hareau, Georges; Kocienski, Philip (2001). "Dimethyl Sulfone". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd371. ISBN 978-0471936237.
- ↑ "Detection of dimethyl sulfone in the human brain by in vivo proton magnetic resonance spectroscopy". Magnetic Resonance Imaging 18 (1): 95–8. January 2000. doi:10.1016/S0730-725X(99)00110-1. PMID 10642107.
- ↑ "Accumulation of methylsulfonylmethane in the human brain: identification by multinuclear magnetic resonance spectroscopy". Toxicology Letters 123 (2–3): 169–77. September 2001. doi:10.1016/S0378-4274(01)00396-4. PMID 11641045.
- ↑ "Dimethyl sulfone in human cerebrospinal fluid and blood plasma confirmed by one-dimensional (1)H and two-dimensional (1)H-(13)C NMR". NMR in Biomedicine 18 (5): 331–6. August 2005. doi:10.1002/nbm.966. PMID 15996001.
- ↑ Jacob, Stanley (2003). MSM the Definitive Guide: Nutritional Breakthrough for Arthritis, Allergies and More. Freedom Press. ISBN 978-1-893910-22-5.[page needed]
- ↑ The Miracle of MSM: The Natural Solution for Pain. New York: Penguin-Putnam. 1999.
- ↑ Lang, Kerry L. (17 June 2001). "Methylsulfonylmethane (MSM)". Quackwatch. http://www.quackwatch.org/01QuackeryRelatedTopics/DSH/msm.html.
- ↑ Herschler RJ, "Dietary and pharmaceutical uses of methylsulfonylmethane and compositions comprising it", US patent granted 4514421, issued 30 April 1985
- ↑ Shanmugam, Srinivasan; Baskaran, Rengarajan; Nagayya-Sriraman, Santhoshkumar; Yong, Chul-Soon; Choi, Han-Gon; Woo, Jong-Soo; Yoo, Bong-Kyu (31 July 2009). "The Effect of Methylsulfonylmethane on Hair Growth Promotion of Magnesium Ascorbyl Phosphate for the Treatment of Alopecia". Biomolecules and Therapeutics 17 (3): 241–248. doi:10.4062/biomolther.2009.17.3.241.
- ↑ "Effects of oral dimethyl sulfoxide and dimethyl sulfone on murine autoimmune lymphoproliferative disease". Proceedings of the Society for Experimental Biology and Medicine 183 (2): 227–30. November 1986. doi:10.3181/00379727-183-42409. PMID 3489943.
- ↑ "Biological effects of the metabolites of dimethyl sulfoxide". Annals of the New York Academy of Sciences 243 (1): 104–9. January 1975. doi:10.1111/j.1749-6632.1975.tb25349.x. PMID 1055534. Bibcode: 1975NYASA.243..104K.
- ↑ "Agency Response Letter GRAS Notice No. GRN 000229". fda.gov. 2008. https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm153891.htm.
- ↑ 20.0 20.1 "MSM Monograph". Therapeutic Research Faculty. https://naturalmedicines.therapeuticresearch.com/databases/food,-herbs-supplements/professional.aspx?productid=522.(Subscription content?)
- ↑ Lim, Eun Joung; Hong, Dae Young; Park, Jin Hee; Joung, Youn Hee; Darvin, Pramod; Kim, Sang Yoon; Na, Yoon Mi; Hwang, Tae Sook et al. (2012). "Methylsulfonylmethane suppresses breast cancer growth by down-regulating STAT3 and STAT5b pathways". PLOS ONE 7 (4): e33361. doi:10.1371/journal.pone.0033361. ISSN 1932-6203. PMID 22485142. Bibcode: 2012PLoSO...733361L.
- ↑ Miller, Lindsey; Thompson, Kari; Pavlenco, Carolina; Mettu, Vijaya Saradhi; Haverkamp, Hans; Skaufel, Samantha; Basit, Abdul; Prasad, Bhagwat et al. (October 2021). "The Effect of Daily Methylsulfonylmethane (MSM) Consumption on High-Density Lipoprotein Cholesterol in Healthy Overweight and Obese Adults: A Randomized Controlled Trial" (in en). Nutrients 13 (10): 3620. doi:10.3390/nu13103620. PMID 34684621.
- ↑ "Toxicity of methylsulfonylmethane in rats". Food and Chemical Toxicology 40 (10): 1459–62. October 2002. doi:10.1016/S0278-6915(02)00086-8. PMID 12387309.
- ↑ Acute oral toxicity of sample No. 751, dimethyl sulfone 1 BT No. A6409. Northbrook, Illinois: Industrial BIO-TEST Laboratories, Inc.. 1968.
- ↑ Ocular and Dermal Irritation Assay for OptiMSM Brand of Methylsulfonylmethane. Grants Pass, Oregon: Flora Research Laboratories. 1999.
- ↑ "Single and 13-week Repeated Oral Dose Toxicity Study of Methylsulfonylmethane in Mice". Oyo Yakuri Pharmacometrics 79: 23–30. 2010.
- ↑ 27.0 27.1 "Efficacy of methylsulfonylmethane (MSM) in osteoarthritis pain of the knee: a pilot clinical trial". Osteoarthritis and Cartilage 14 (3): 286–94. March 2006. doi:10.1016/j.joca.2005.10.003. PMID 16309928.
- ↑ 28.0 28.1 "Efficacy of methylsulfonylmethane supplementation on osteoarthritis of the knee: a randomized controlled study". BMC Complementary and Alternative Medicine 11: 50. June 2011. doi:10.1186/1472-6882-11-50. PMID 21708034.
- ↑ 29.0 29.1 Pagonis, Thomas A (2014). "The Effect of Methylsulfonylmethane on Osteoarthritic Large Joints and Mobility". International Journal of Orthopaedics 1 (1): 19–24. doi:10.6051/j.issn.2311-5106.2014.01.7. http://www.ghrnet.org/index.php/ijo/article/view/745/862.
- ↑ Bauer, Brent A. (6 June 2014). "MSM for arthritis pain: Is it safe?". Expert Answers. Mayo Clinic. http://www.mayoclinic.com/health/msm/AN00560.
- ↑ 31.0 31.1 "Randomised, Double-Blind, Parallel, Placebo-Controlled Study of Oral Glucosamine, Methylsulfonylmethane and their Combination in Osteoarthritis". Clinical Drug Investigation 24 (6): 353–63. 2004. doi:10.2165/00044011-200424060-00005. PMID 17516722.
- ↑ "Systematic review of the nutritional supplements dimethyl sulfoxide (DMSO) and methylsulfonylmethane (MSM) in the treatment of osteoarthritis". Osteoarthritis and Cartilage 16 (11): 1277–88. November 2008. doi:10.1016/j.joca.2008.03.002. PMID 18417375.
- ↑ Alekseeva, L.I.; Sharapova, E.P.; Kashevarova, N.G.; Taskina, E.A.; Anikin, S.G.; Korotkova, T.A.; Pyanykh, S.E. (2015). "Use of ARTRA MSM FORTE in patients with knee osteoarthritis: Results of a randomized open-label comparative study of the efficacy and tolerability of the drug". Therapeutic Archive 87 (12): 49–54. doi:10.17116/terarkh2015871249-54. PMID 26978418.
- ↑ Lubis, Andri M T; Siagian, Carles; Wonggokusuma, Erick; Marsetyo, Aldo F; Setyohadi, Bambang (2017). "Comparison of Glucosamine-Chondroitin Sulfate with and without Methylsulfonylmethane in Grade I-II Knee Osteoarthritis: A Double Blind Randomized Controlled Trial.". Acta Medica Indonesiana 49 (2): 105–111. PMID 28790224. http://www.actamedindones.org/index.php/ijim/article/view/168/pdf. Retrieved 10 April 2020.
- ↑ Anthonavage, Michael; Benjamin, Rod; Withee, Eric (2015). "Effects of oral supplementation with methylsulfonylmethane on skin health and wrinkle reduction". Natural Medicine Journal 7 (11): 1–21. https://www.naturalmedicinejournal.com/journal/2015-11/effects-oral-supplementation-methylsulfonylmethane-skin-health-and-wrinkle-reduction.
- ↑ Muizzuddin, Neelam; Benjamin, Rodney (2020-02-21). "Beauty from within: Oral administration of a sulfur-containing supplement methylsulfonylmethane improves signs of skin ageing" (in en). International Journal for Vitamin and Nutrition Research 92 (3–4): 182–191. doi:10.1024/0300-9831/a000643. ISSN 0300-9831. PMID 32083522.
- ↑ Muizzuddin, Neelam; Benjamin, Rodney (2019). "Beneficial Effects of a Sulfur-Containing Supplement on Hair and Nail Condition". Natural Medicine Journal 11 (11): 1–8. https://www.naturalmedicinejournal.com/journal/2019-11/beneficial-effects-sulfur-containing-supplement-hair-and-nail-condition.
- ↑ Guaitolini, Ennio; Cavezzi, Attilio; Cocchi, Stefania; Colucci, Roberto; Urso, Simone Ugo; Quinzi, Valentina (2019). "Randomized, Placebo-controlled Study of a Nutraceutical Based on Hyaluronic Acid, L-carnosine, and Methylsulfonylmethane in Facial Skin Aesthetics and Well-being". The Journal of Clinical and Aesthetic Dermatology 12 (4): 40–45. ISSN 1941-2789. PMID 31119010.
- ↑ "Effect of methylsulfonylmethane supplementation on exercise - Induced muscle damage and total antioxidant capacity". The Journal of Sports Medicine and Physical Fitness 52 (2): 170–4. April 2012. PMID 22525653. http://www.minervamedica.it/index2.t?show=R40Y2012N02A0170.
- ↑ "Effect of chronic supplementation with methylsulfonylmethane on oxidative stress following acute exercise in untrained healthy men". The Journal of Pharmacy and Pharmacology 63 (10): 1290–4. October 2011. doi:10.1111/j.2042-7158.2011.01314.x. PMID 21899544. http://eprints.arums.ac.ir/2588/1/chronic.pdf.
- ↑ "Effect of single dose administration of methylsulfonylmethane on oxidative stress following acute exhaustive exercise". Iranian Journal of Pharmaceutical Research 12 (4): 845–53. 2013. PMID 24523764.
- ↑ "Effect of Methylsulfonylmethane Pretreatment on Aceta-minophen Induced Hepatotoxicity in Rats". Iranian Journal of Basic Medical Sciences 16 (8): 896–900. August 2013. PMID 24106592.
- ↑ "Effect of methylsulfonylmethane on paraquat-induced acute lung and liver injury in mice". Inflammation 36 (5): 1111–21. October 2013. doi:10.1007/s10753-013-9645-8. PMID 23595869.
- ↑ "Hepatoprotective effect of methylsulfonylmethane against carbon tetrachloride-induced acute liver injury in rats". Archives of Pharmacal Research 36 (9): 1140–8. September 2013. doi:10.1007/s12272-013-0110-x. PMID 23591777.
- ↑ Disilvestro, Robert A; Disilvestro, David J; Disilvestro, Daniel J (2008). "Methylsulfonylmethane (MSM) Intake in Mice Produces Elevated Liver Glutathione and Partially Protects Against Carbon Tetrachloride-Induced Liver Injury". FASEB J. 22 (1): 445–8. doi:10.1096/fasebj.22.1_supplement.445.8.
- ↑ "The effect of methylsulfonylmethane on the experimental colitis in the rat". Toxicology and Applied Pharmacology 253 (3): 197–202. June 2011. doi:10.1016/j.taap.2011.03.017. PMID 21463646.
- ↑ "Protective effects of methylsulfonylmethane on hemodynamics and oxidative stress in monocrotaline-induced pulmonary hypertensive rats". Advances in Pharmacological Sciences 2012: 1–6. 2012. doi:10.1155/2012/507278. PMID 23118745.
- ↑ Barrager, Eleanor; Veltmann, Joseph R.; Schauss, Alexander G.; Schiller, Rebecca N. (2002). "A Multicentered, Open-Label Trial on the Safety and Efficacy of Methylsulfonylmethane in the Treatment of Seasonal Allergic Rhinitis" (in en). The Journal of Alternative and Complementary Medicine 8 (2): 167–173. doi:10.1089/107555302317371451. ISSN 1075-5535. PMID 12006124.
- ↑ Hewlings, Susan; Kalman, Douglas S (2018-11-29). "Evaluating the Impacts of Methylsulfonylmethane on Allergic Rhinitis After a Standard Allergen Challenge: Randomized Double-Blind Exploratory Study" (in en). JMIR Research Protocols 7 (11): e11139. doi:10.2196/11139. ISSN 1929-0748. PMID 30497995.
- ↑ van der Merwe, Mariè; Bloomer, Richard J. (2016). "The Influence of Methylsulfonylmethane on Inflammation-Associated Cytokine Release before and following Strenuous Exercise" (in en). Journal of Sports Medicine 2016: 7498359. doi:10.1155/2016/7498359. ISSN 2356-7651. PMID 27844051.
- ↑ Kim, Yoon Hee; Kim, Dae Hwan; Lim, Hwan; Baek, Doo-Yeon; Shin, Hyun-Kyung; Kim, Jin-Kyung (2009). "The anti-inflammatory effects of methylsulfonylmethane on lipopolysaccharide-induced inflammatory responses in murine macrophages". Biological & Pharmaceutical Bulletin 32 (4): 651–656. doi:10.1248/bpb.32.651. ISSN 0918-6158. PMID 19336900.
- ↑ Karabay, Arzu Z.; Aktan, Fugen; Sunguroğlu, Asuman; Buyukbingol, Zeliha (2014). "Methylsulfonylmethane modulates apoptosis of LPS/IFN-γ-activated RAW 264.7 macrophage-like cells by targeting p53, Bax, Bcl-2, cytochrome c and PARP proteins" (in en). Immunopharmacology and Immunotoxicology 36 (6): 379–389. doi:10.3109/08923973.2014.956752. ISSN 0892-3973. PMID 25211405.
 |

