Chemistry:Silver trifluoromethanesulfonate
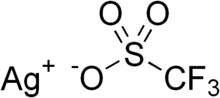
| |
| Error creating thumbnail: Unable to save thumbnail to destination | |
| Names | |
|---|---|
| IUPAC name
silver trifluoromethanesulfonate
| |
| Other names
Silver triflate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| Abbreviations | AgOTf |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| CF3SO3Ag | |
| Molar mass | 256.937 g/mol |
| Odor | odorless |
| Melting point | 286 °C (547 °F; 559 K) |
| soluble | |
| Hazards | |
| Safety data sheet | Oxford MSDS |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H315, H319, H335 | |
| P260, P261, P264, P271, P280, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P332+313, P337+313, P362, P363, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Silver trifluoromethanesulfonate, or silver triflate is the triflate (CF3SO3−) salt of Ag+. It is a white or colorless solid that is soluble in water and some organic solvents including, benzene. It is a reagent used in the synthesis of organic and inorganic triflates.
Synthesis
An early preparation method starts from the barium salt of trifluoromethanesulfonic acid (TfOH), from which the free TfOH is formed with dilute sulfuric acid, which is then neutralized with silver carbonate (Ag2CO3).[2][3]
The silver triflate is thereby obtained in a yield of 95% and can be recrystallized from benzene/tetrachloromethane or ether/tetrachloromethane for purification.
In an improved version by George Whitesides, dilute TfOH is reacted with silver(I)oxide (Ag2O), which produces AgOTf in 98% yield.[4]
Reactions
It is used to prepare alkyl triflates from alkyl halides:[5]
- CF3SO2OAg + RX → CF3SO2OR + AgX (X = iodide usually)
In coordination chemistry, the salt is also useful to replace halide ligands with the more labile triflate ligand. For example, bromopentacarbonylrhenium can be converted to the more labile derivative using silver triflate:[6]
- CF3SO2OAg + BrRe(CO)5 → CF3SO2ORe(CO)5 + AgBr
References
- ↑ "Silver trifluoromethanesulfonate" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/76223#section=Safety-and-Hazards.
- ↑ R.N. Haszeldine, J.M. Kidd (1954), "Perfluoroalkyl derivatives of sulphur. Part I. Trifluoromethanesulphonic acid" (in German), J. Chem. Soc.: 4228–4232, doi:10.1039/JR9540004228
- ↑ T. Gramstadt, R.N. Haszeldine (1956), "33. Perfluoroalkyl derivatives of sulphur. Part IV. Perfluoroalkanesulphonic acids" (in German), J. Chem. Soc.: 173–180, doi:10.1039/JR9560000173
- ↑ G.M. Whitesides, F.D. Gutowski (1976), "Reaction of α, ω-di-Grignard reagents with silver(I) salts form carbocyclic rings", J. Org. Chem. 41 (17): 2882–2885, doi:10.1021/jo00879a019
- ↑ Stang, Peter J.; Hanack, Michael; Subramanian, L. R. (1982). "Perfluoroalkanesulfonic Esters: Methods of Preparation and Applications in Organic Chemistry". Synthesis 1982 (2): 85–126. doi:10.1055/s-1982-29711. ISSN 0039-7881.
- ↑ Steven P. Schmidt; Jay Nitschke; William C. Trogler (1989). "Manganese(I) and Rhenium(I) Pentacarbonyl(Trifluoromethanesulfonato) Complexes". Inorganic Syntheses. 26. pp. 113–117. doi:10.1002/9780470132579.ch20. ISBN 978-0-470-13257-9.
 |


